eOrganic authors:
Patrick Merscher, Culinary Breeding Network
Alex Stone, Oregon State University
Table of Contents
- Adjuvant Introduction: What Are They, and Why Use Them?
- Activator Adjuvants
- Surfactants
- Nonionic Surfactants
- Organosilicons
- Biosurfactants
- Glycosides (Saponins, Rhamnolipids, MELs)
- Oils and Lipids
- Utility Adjuvants
- Buffers, Acidifiers, and Conditioning Agents
- De/Antifoam Agents
- Wetters, Spreaders, Emulsifiers
- Deposition Aids, Stickers, Weatherproofing Agents
- Dyes, Markers, Colorants
- Drift Control Agents, Thickeners
- Using an Adjuvant on Your Farm: Adjuvant Registration, Regulation, and Organic Compliance
- Choosing an Adjuvant
- A Realistic (but Hypothetical) Example for Using this Guide
Adjuvant Introduction: What Are They, and Why Use Them?
Adjuvant comes from the Latin adjuvare, which means to help. It is a relatively broad term referring to substances that are added to pesticides or nutrient solutions for the purpose of improving their mixing, application, or effectiveness (see Table 1). Some product formulations already contain one or multiple adjuvants, while other adjuvants can be purchased to add into tank mixes before application. IMPORTANT: Before using any pest control product in your organic farming system:
- Read the label to be sure that the product is labeled for the crop and pest you intend to control, and make sure it is legal to use in the state, county, or other location where it will be applied.
- Read and understand the safety precautions and application restrictions.
- Make sure that the brand name product is listed in your Organic System Plan and approved by your USDA-approved certifier. If you are trying to deal with an unanticipated pest problem, get approval from your certifier before using a product that is not listed in your plan—doing otherwise may put your certification at risk.
Note that OMRI and WSDA lists are good places to identify potentially useful products, but all products that you use must be approved by your certifier. For more information on how to determine whether a pest control product can be used on your farm, see the article, Can I Use This Input On My Organic Farm?
There are many reasons to use an adjuvant, including but not limited to:
- improve droplet spread and reduce potential for drops to roll off leaves
- boost effectiveness of spray's active ingredients
- reduce quantity of active ingredient applied, lowering costs and environmental impact
- reduce quantity of water needed in tank
- promote better mixing and distribution of active ingredients in solution
- enhance persistence and weatherability of active ingredients on leaves
- prevent evaporation or UV degradation
- enhance penetration/absorption of active ingredients into plants
- alter solution pH or water hardness to improve effectiveness
Pesticides have to overcome a variety of obstacles in order to be effective. For example, active ingredients in contact pesticides need time to work, but aqueous spray solutions may bead up and/or roll off plant leaves, making them less effective. Leaf hairs, cuticular waxes, and foaming in the tank can also prevent good contact of active ingredients with their target pests and plant tissues. Good contact between the spray solution and the plant is necessary for penetration of active ingredients into the plant body. Adjuvants can help overcome these barriers. They minimize spray application problems and boost the spray's effectiveness if used appropriately. When spraying, using a recommended adjuvant can boost spray efficacy on average of 30-50%, but incompatible or inappropriate use of adjuvants can cause or exacerbate problems like phytotoxicity (Czarnota and Thomas, 2013).
| Table 1: Adjuvant Types for Organic Pest/Disease Management and Control | |
| Adapted from Tu and Randall (2003) |
|
| Activator adjuvants (boost a: | Utility adjuvants: |
| Surfactants | Wetting agents (wetters, spreaders) |
| -Nonionic | Dyes and Pigments |
| -Organosilicons | Drift/foam control |
| Biosurfactants (Glycosides and others) | Thickening agents |
| -Soaps, saponins | Deposition agents (stickers) |
| -Microbial-derived (rhamnolipids, MELs) | pH Buffers, Acidifiers |
| Oils and Lipids | Humectants, Penetrants |
| -Plant derived | UV absorption/protection/Weatherproofing |
Table 1: Activator and utility adjuvants. Activator adjuvants boost a spray's activity, while utility adjuvants fix spray/mixing problems.
Activator Adjuvants
Activator adjuvants are so named because they enhance the activity of a spray, referring to its active ingredients. The improvements—both chemical and physical —generally enhance coverage (wetting/spreading) and can increase absorption of the active ingredient by plants, thereby making the spray more effective. Absorption or penetration of active ingredients can be achieved by altering leaf tissue permeability, lengthening the amount of time a solution stays wet (available), or encouraging entry through natural openings like stomata.
Surfactants
Compounds that alter surface tension are called surfactants (a contraction of surface acting agents). Surfactants have a hydrophilic head (water-loving) and a hydrophobic or lipophilic tail (water-hating/oil-loving). Because of this structure, surfactants reduce the internal energy (surface tension) of a spray solution droplet. The surface tension of water is created by hydrogen bonds between water molecules, and this internal energy forms the spray solution droplet (mostly water) into a perfect sphere. The spherical (bead) shape of the droplet means that little of the bead (spray solution) is in direct contact with the leaf, and beads can easily roll off. Surfactant molecules are both water and oil soluble, which allows them to move to the surface of the liquid and disrupt the hydrogen bonds between water molecules. This reduces surface tension and flattens the droplet (Fig. 1), allowing it to cover more of the leaf surface and reducing the potential for rolling off. Surface tension is measured in dynes/cm. The surface tension of water is typically 72 dynes/cm, and a good surfactant should reduce the surface tension of the spray solution to 20-30 dynes/cm (Brandt Consolidated, 2016).
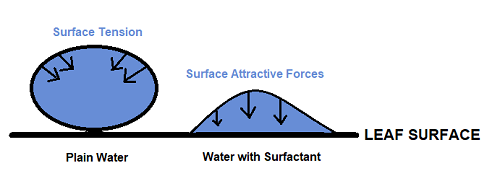
Figure 1: Surfactants alter surface tension to enhance spreading of droplets. Surface tension of water causes it to bead up and roll off leaves. Surfactant adjuvants decrease surface tension and enhance spreading and retention of the spray solution on the leaf. Drawn by author.
As a group, surfactants are often categorized by the way they ionize (break apart and form charged particles in water). Cationic (positively charged) surfactants are almost never used in agriculture as they are very phytotoxic.
Anionic (negatively charged) surfactants are commonly used with contact pesticides in conventional agriculture and some (e.g. sodium lauryl sulfate, commonly used in shampoos) are listed as Inerts of Minimum Concern. The USDA National Organic Program (NOP) includes these ingredients in the National List of Allowed and Prohibited Substances as long as they are being used as an inert mixed with an approved pesticide (CFR 7 §205.601). It cannot be emphasized enough, before using any input on your organic farm, check with your certifier and add the product to your Organic Systems Plan. Despite being allowed, at the time of this writing, no anionic surfactants for crop production were found in the OMRI database. However, it is possible some pesticide formulations contain them (often as sulfates), since inert ingredients are not required to be listed on the label (or they might be listed with a different organic input reviewer). Anionic surfactants are common in detergents and cosmetic products. They cause heavy foaming and mild phytotoxicity (Czarnota and Thomas, 2013). The presence of anionic and cationic surfactants in household detergents and cleaners is one reason why homemade recipes (frequently suggested on the Internet) should be avoided for farm and garden use due to the potential for phytotoxicity and heavy foaming.
Nonionic Surfactants
Nonionic (neutral charge) surfactants do not form ions in water and are commonly used in agriculture. There are many allowed for use in organic production. Typically, nonionic surfactants are alcohols and/or fatty acids from both natural and synthetic sources. Their basic structure is depicted in Figure 2. Their tails are often long hydrocarbons (chains of carbon and hydrogen atoms). They vary in the length of the chain and in the number of double bonds (saturation); these differences partly explain the variability in their adjuvant functions (Northover and Timmer, 2002).
Nonionic surfactants are the most recommended ones for use with registered pesticides because they are compatible with a wide range of pesticidal activities (systemic, contact, etc.)(Hock et al., 2016). In addition, they aid in absorption of active pesticide ingredients without causing tissue damage (Czarnota and Thomas, 2013), and they can be used under a wide range of environmental conditions.
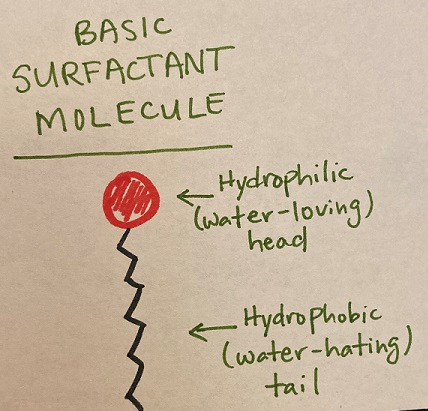
Figure 2: The structure of a basic surfactant molecule. They have a hydrophilic head group and a hydrophobic tail. There is great structural, chemical, and functional diversity among surfactants, but they share this basic structure. Drawn by author.
OMRI/WSDA listed examples: Nonionic Surfactants
| Product | Manufacturer |
| Biolink Spreader-Sticker | Westbridge |
| NanoWet Premium | Green Vision Life Sciences |
| Kinetic | Helena Agri-Enterprises |
| Oroboost, OroRZ | Oro Agri |
Organosilicons
Organosilicons, as implied in their name, contain atoms of silicon in their hydrophobic tails; this makes them more flexible (Hill, 1999) and therefore more effective (on average, 10 dyne/cm more) in reducing surface tension than ionic and nonionic surfactants. Figure 3 compares organosilicon surfactant tails with hydrocarbon (nonionic) surfactant tails.
Organosilicons used as adjuvants are generally made from silica (silicon dioxide), one of the most abundant molecules in Earth's crust. Some are considered naturally-occurring, while others are considered synthetic based on the NOP's Guidance 5033-1. Naturally-produced organosilicon products can be used in organic systems. Synthetic silicones and silicas are prohibited for organic crop production use. The synthesis or manufacturing process is what determines which category an organosilicon falls into, and there are many products listed in the OMRI database.
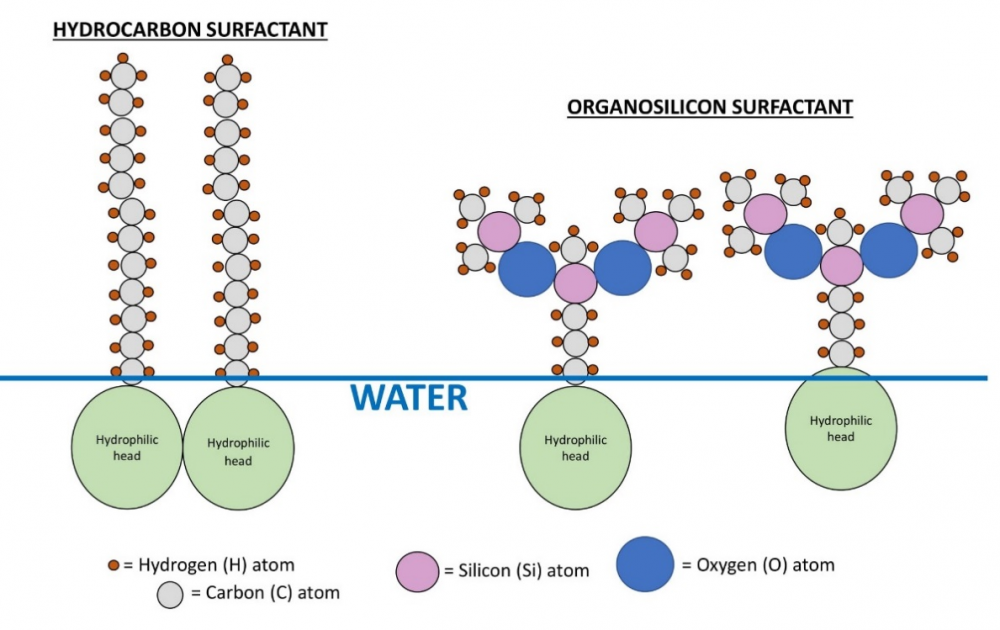
Figure 3: Hydrocarbon surfactant tails vs. organosilicon. A comparison of hydrocarbon surfactant tails (left) vs. organosilicon surfactant tails (right). The shaded circles represent hydrophilic heads. The O-Si backbone gives organosilicon surfactants more flexibility and better ability to reduce surface tension. Drawn by author.
Some organosilicon surfactants demonstrate a phenomenon known as superspreading or super wetting. Superspreading represents the extreme end of surfactant outcomes, in which the droplet completely disappears, and the spray solution spreads out in a thin layer across the leaf surface. The solution can become so fluid it flows right off the leaf, defeating the purpose of the adjuvant. This is generally a function of the specific organosilicon and its concentration in the solution. The superspreading activity of organosilicons has been associated with better rainproofing, which is explained by an increase in stomatal entry of active ingredients (Pacanoski, 2015). Too-high surface tension or droplets that are too large cause little to no contact between the spray active ingredients and stomates. Superspreading enhances the contact area, and thereby the penetration of spray ingredients into the leaf, which increases its persistence to outlast the rain. Organosilicons should only be applied at temperatures below 90°F as they can cause phytotoxicity at high temperatures.
OMRI/WSDA listed examples: Organosilicon Surfactants
| Product | Manufacturer |
| Vestis | Loveland Products |
| Break-Thru | Evonik Co. |
| SP TWEEN 22 | Croda |
| Brandt 719 Spreader | Brandt |
| Broadspred Green | Custom Ag Formulators |
| VistaSil 100 | Mar Vista Resources |
| Matrixx | Coastal Agro Business |
Biosurfactants
Biosurfactants are (usually) microbial metabolites with surfactant properties, and they fall into four main categories: lipopeptides, glycolipids (see glycosides), phospholipids (cell membranes), and polymeric compounds (Liu et al., 2015). The biosurfactants described below are those most relevant as organic agriculture adjuvants.
Glycosides (Saponins, Rhamnolipids, MELs)
Glycosides are a diverse molecular group. Chemically, they consist of a sugar molecule bound to another functional group via glycosidic linkage. Many plants store inactive glycosides in their cells, but these become activated by stress and/or herbivory signals and can act as defense compounds (Hussain et al., 2019). Interestingly, some people have figured out how to use these ecological relationships to the farmer's advantage. For example, curcurbitacins can act as feeding stimulants that cause uncontrollable eating behavior of insects. When added to pesticide sprays, these feeding stimulants reportedly boost the pesticide's effectiveness because insects ingest more (Trece, Inc., 2012).
Saponins are a distinct yet diverse group of glycosides used in organic agriculture as biosurfactants. Saponins are sometimes termed soaps (as they foam when agitated) on product labels. This can be confusing, as ionic surfactants (detergents) can also be termed soaps. When a product is described as containing 100% soap, carefully read the product's label and Material Safety Data Sheet (MSDS or SDS) to understand what the product's ingredients are and what that means for product function.
Saponin adjuvants are derived from diverse plants including soapbark (Quillaja saponaria), yucca (Yucca schidigera), tea seed (Camellia oleifera), and quinoa (Chenopodium quinoa) (Zhu et al., 2010). Considered non-synthetic, saponins are allowed in organic systems, while other soaps have restrictions. Products can come as wettable powders or as liquids. They function as surfactants, reducing the surface tension of the spray solution. Some saponin products are marketed as having multiple uses, for example as both a pesticide and an adjuvant. If a product makes claims about pesticidal activity, it must be registered as a pesticide. There is growing concern about the toxicity of saponins on non-target species such as crustacean and fish embryos (Jiang et al., 2019). They can also react with certain pesticides (particularly copper fungicides) and cause phytotoxicity (Caldwell et al., 2013).
Microbially-derived glycosides are growing in popularity. The two most common are rhamnolipids (usually produced by the bacteria Pseudomonas aeruginosa) and mannosylerythritol lipids (MELs) (produced by a variety of yeasts and fungi including Pseudozyma sp. and Ustilago sp.). They function like saponins and other surfactants by helping polar and nonpolar substances mix, and reducing surface tension at the contact interface. Despite being discovered in the 1950s, the application of rhamnolipids and MELs as adjuvants is somewhat new in organic agriculture (Arujo et al., 2018). These biosurfactants are a burgeoning area of research and development because they are effective at low concentrations, thought to be less toxic to non-target species, and the microbes can be grown on recycled material (e.g., spent cooking oil).
OMRI/WSDA listed examples: Biosurfactant Adjuvants
| Type | Product | Manufacturer |
| MELs | RadiaSurf ML | Oleon Americas |
| Rhamnolipids | JBR 425 | Jeneil Biotech |
| Soap/Saponin | Spread Coat | Flying Skull |
|
| CleanGreen I.F.C. Adjuvant | U.S. Ag., LLC |
|
| Ampersand | Attune |
|
| OY-1S Organic Adjuvant | O2YS Corporation |
|
| Protector | Henry Manufacturing |
Oils and Lipids
Oils and lipids are not surfactants; they are nonpolar hydrocarbons. They are often composed of fatty acids bound to some type of backbone like glycerol. The molecular behavior of oils as adjuvants has not been well-studied, unlike their use as a pesticide (Caldwell et al., 2013). They come in a variety of forms and chemical structures that can be derived from diverse sources. They usually require or are formulated with about 20% nonionic surfactant as an emulsifier (Hock et al., 2016).
Oils are used for a wide range of purposes in organic agriculture. They are commonly used as pesticides to control soft-bodied insects (e.g., mites, aphids, whiteflies, thrips, mealybugs, and scale) and fungal diseases (e.g., powdery mildew) (Caldwell et al., 2013). Oils and lipids can smother spores, hyphae, eggs, and insect bodies thereby disrupting normal gas exchange. They are also commonly used in orchards for fruit/blossom thinning, and they have been shown to induce plant systemic immune responses, although more research is needed (Northover and Timmer, 2002).
Organic farmers use oils as adjuvants to increase the oil-phase concentration of the spray solution. In simpler terms, oils add more hydrophobic material into the mix. This affects the dispersal and interactions of spray ingredients; for example, oils will interact with surfactant tails and hydrophobic ingredients in the pesticide. After application, oils help prevent sprays from evaporating and give active ingredients more time to penetrate and work (Tu and Randall, 2003). Some oils have also been shown to modify characteristics of leaf cuticular waxes (i.e., causing them to crack) which enhances penetration into the plant (Tu and Randall, 2003). There is relatively little information published on this subject, although many chemical companies own related patents. Historically, oils are common adjuvants with herbicides targeting grasses, as they seem to be particularly good at enhancing penetration of active ingredients into waxy monocot leaves (Tu and Randall, 2003).
Phytotoxicity is frequently reported with oil use and is exacerbated by hot and humid temperatures, which is one reason why they are most used in orchards during winter dormancy (Caldwell et al., 2013). Negative effects of oil use include tip burn on leaves and decreases in fruit yield or quality (i.e., soluble sugars) (Northover and Timmer, 2002; Caldwell et al., 2013). Phytotoxicity can be mitigated by ensuring appropriate emulsification and dilution in the tank and applying when temperatures are below 80°F (Caldwell et al., 2013). Oils can be incompatible with some products, such as sulfur and copper fungicides; always be sure to read labels.
Vegetable oils are pressed from seeds like soybeans, sunflower, and canola. The NOP allows their use with the restriction that the source material cannot be produced with excluded methods such as genetic engineering or ionizing radiation (CFR 7 §205.601). Esterification of vegetable oils produces a methylated seed oil; this process increases the oil's interaction with the oil-soluble active ingredients in pesticides and aids in distribution throughout the solution. Cold-pressed, raw/unaltered, or mixtures of vegetable oils can contain a diversity of lipid types and structures which may enhance functional diversity (Liu et al., 2015), but raw or mixed oils may also have unpredictable consistency and functionality in spray mixtures because of natural variation in source materials.
Most petroleum-based oil adjuvants are synthetic and not allowed in organic systems. However, one exception in the NOP is narrow–range petroleum oils allowed for pest and disease control (Caldwell et al., 2013). Crude oil is heated, which causes it to separate into different fractions. Each fraction is composed of hydrocarbons of specific molecular weights, structures, and activities. Narrow-range oils, as defined by the NOP, have a median boiling point of 415-440°F under vacuum conditions. This fraction of oil has enough persistence to be effective on pests but causes a low degree of phytotoxicity (Caldwell et al., 2013). Unlike nonionic and anionic surfactants (which have to be used as inerts), narrow-range petroleum oils must be used as an active ingredient (i.e., as a pesticide) in organic systems, but they have some natural adjuvant properties when present in a mix; for example, they can cause fissures in leaf cuticles (Pacanoski, 2015).
OMRI/WSDA-listed examples: Oil and Lipid Adjuvants
| Product | Manufacturer |
| Crocker’s Fish Oil Spreader Sticker | Crocker’s Fish Oil, LLC |
| EcoBlend Adjuvant | HOMS, LLC |
| Karanja Pro | Neem Pro |
| Surfynol 485/485W | Evonik |
Utility Adjuvants
Utility adjuvants fix conditions that would otherwise negatively affect the spray solution or its application. By improving mixing and application, the spray's effectiveness is likewise upgraded. Buffers, conditioners, acidifiers, compatibility agents, de/antifoam agents, and thickeners change the characteristics of the spray solution. Humectants, dyes, stickers, rainproofers, and UV protectants fight against unfavorable environmental conditions and visually assist the applicator. Other types of dyes, markers, and equipment cleaning agents can be included in the utility adjuvant category, since clean equipment also improves spray quality. Be mindful that organic food/product processing similarly requires the use of approved adjuvants, so be sure the product you purchase is made and tested for agricultural situations.
Buffers, Acidifiers, and Conditioning Agents
Most pesticides perform best in an acidic solution (pH 4.5—6.5). When the pH is too alkaline, a pesticide can lose half its effectiveness in as little as 15 minutes (Hock et al., 2016). Acidifiers lower the pH of the mixing water, but they may not necessarily hold the pH constant. Some acidifier products come with a dye that turns color when the solution is the appropriate pH. Buffers are usually weak acids or bases that help keep pH constant in a solution. If an acidifier or buffer is added, it is usually recommended to test the pH throughout mixing and even once or twice during application, especially if application takes more than two hours. The most common acidifiers are forms of citric acid such as β-hydroxytricarballylic acid with additional additives like calcium chloride or molasses. Be sure to check the pesticide label for warnings about potential reactions, especially with copper and sulfur sprays.
Conditioning agents (sometimes called complexing agents) are also available adjuvants. Minerals in hard water (mostly calcium and magnesium) can react with nutrient solutions and pesticide ingredients to precipitate and form insoluble compounds. Water softeners and other conditioning agents prevent this reaction. These interventions can be physical (for example, carbon or magnetic filters) or chemical (enzymatic or chelators).
OMRI/WSDA-listed examples: Buffers, Acidifiers, and Conditioning Agents
| Product | Manufacturer |
| Nature’s Nectar Zyme and Enzymatic Complex Water Conditioner | EZ Gro |
| pH Down | SafeGro Laboratories |
| MixWell Acidifier | JH Biotech |
| Tri-Fol | Wilbur-Ellis |
| CitriSan Citric Acid | 02YS Corporation |
| Aqua Buff | Mar Vista Resources |
| BB5 NC | NutriAg |
| Constant BUpHER | Brandt |
De/Antifoam Agents
Foam is caused by air bubbles trapped in the spray liquid, and it is often exacerbated by agitation and stabilized by surfactants. These air bubbles are undesirable because they can cause patchy, uneven spray coverage and cause equipment clogging. Antifoam agents prevent the formation of foam, whereas defoamers work to destroy foam that has already formed. Their modes of action are essentially the same—one is preventative though and the other is not—and the two terms are often used interchangeably. Most organic foam control products are silicon-based with label ingredients like silica, siloxanes, and/or silicone dioxide.
OMRI/WSDA-listed examples: Anti-/Defoamers
| Product | Manufacturer |
| Brandt Defoamer | Brandt |
| Elimino | Precision Laboratories |
| Sipernat 22/22S/340/350 | Evonik |
| De-Foamer OR-10 | Chemurgic |
Wetters, Spreaders, Emulsifiers
Wetters, spreaders, and emulsifiers are common descriptors for surfactant products, as described above. Emulsifiers help nonpolar ingredients and water mix in the tank—another property of surfactants. When mixed in an aqueous solution, surfactant molecules aggregate to form micelles, which are spherical, oblong, or bilayer structures that organize so their hydrophilic heads face the surrounding water with their hydrophobic tails on the inside (see Fig. 4). Micelle formation is affected by the surfactant's specific chemistry, ambient temperature, atmospheric pressure, and concentration of surfactant in solution (McClements, 2012). The micelles form pockets (or cores) where other hydrophobic substances (like active ingredients in pesticides) get trapped. In a tank solution, the hydrophilic heads of surfactant molecules interact with the surrounding water. This helps distribute the pesticide in micelles throughout the solution.
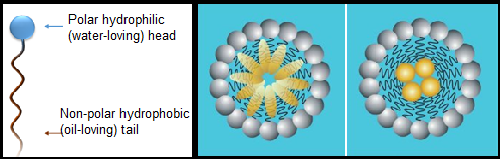
Figure 4: Micelle Formation. Surfactant molecules form micelles with their hydrophobic tails forming a pocket or core that traps other oil-soluble material like active pesticide ingredients. Figure from McClements, 2012.
Another type of wetting agent prevents the crystallization of active ingredients that occurs with evaporation, thereby lengthening the amount of time a leaf surface stays wet. Keeping the leaf wet allows more time for contact pesticides to work and time for penetration through leaf cuticles and stomata. For example, humic acids and glycerol can act as humectants—a substance that attracts and holds water molecules like a magnet (sometimes called hygroscopic). Polymers and weatherproofing agents can also slow the effects of natural drying processes.
OMRI/WSDA-listed examples: Wetters, Spreaders, Emulsifiers
| Product | Manufacturer |
| Synperonic PE/F 127 | Croda |
| Brandt Super Wetter | Brandt |
| Leaf Life, Freeway | Loveland Products |
| Silwet ECO Spreader, Silwet 719 Spreader, Agrospred Flex | Momentive Performance Materials |
Deposition Aids, Stickers, Weatherproofing Agents
Deposition aids are also called stickers, and they can have a few different functions. In general, stickers help pesticide particles stay on the plant surface, and in many cases is accomplished by weatherproofing the spray solution. Weatherproofing agents protect the spray from washing off by irrigation or rain, and from photodegradation by UV rays. Stickers can also increase droplet size, which reduces droplets bouncing off leaves (see Drift Control Agents). Sticker products often contain a wetting agent (surfactant) and are therefore marketed as spreader-stickers.
Polymers are a common type of adjuvant approved for use in organic farming. They are commonly derived from pinene, which is an aromatic terpene contributing to pine tree aromas. They can be added to spray solutions as weatherproofing agents, stickers, penetrants, and drift reducers depending on their concentration. For solutions/products using polymers as a weatherproofing agent, sticker, or penetrant, a short (~30 minutes) drying period is required after application for polymers to coalesce and form a film (see Fig. 5). The film protects the spray's active ingredients by slowing evapotranspiration (helps keep things wet and penetrate) and preventing wash off and volatilization (helps keep things in place) (Borjesson, 2010).
Figure 5: Polymer Film Coalescing over Spray Droplets on Leaf: After time to allow setting, polymers coalesce and form films to protect spray droplets from weather and degradation. Drawn by author.
OMRI/WSDA-listed examples: Weatherproofing and Polymer Adjuvants
| Product | Manufacturer |
| Sustain, NuFilm P | Miller Chemical and Fertilizer |
| PolyHydra-O | Synbiotic Evolution |
| ArgosyO Rain-Fast | Lidochem, Inc. |
| Agrilan 755 | Nourvon |
| Mineral Tech CM Zeolite 100% Pure Spray/Sticker Adjuvant/UV Blocker | Agroenvironment |
| Piccolyte AO Plus | Pinova Solutions |
Dyes, Markers, Colorants
Dyes, markers, or colorants may be added to spray mixes for two general reasons. First, pigments can prevent photodegradation of active ingredients, so they can work as a weatherproofing aid (Pacanoski, 2015). Second, dyes and markers can be added to assist the applicator. For example, pH-activated dyes signal when a solution is the appropriate pH. Dyes may also be added to show the applicator where the spray solution has (and has not) been applied or deposits in tanks/equipment that need to be cleaned. It is illegal to add food coloring or other household products to tank mixes; only products designed, formulated, and registered for agricultural purposes can be applied to crops.
Many products designed and approved for organic use are made from complex biological materials; for these materials, the color is derived from natural pigments. Molasses, for example, contains melanoidins that give it a dark brown color, and these have been shown to protect against UV damage and evaporation (Graber et al., 2015). The biological complexity of these materials can provide benefits as well as costs. For example, while molasses has built-in UV protection, it can also act as a food source for pests and pathogens (Hock et al., 2016).
Drift Control Agents, Thickeners
Spray drift poses environmental and human health risks. It is of particular concern around sensitive areas and when environmental conditions are not conducive to sprays (excessive winds). In organic farming, the topic of drift usually centers around detrimental effects of neighboring conventional farmers and their sprays, but organic farmers should also reduce the risk of pesticide applications drifting onto non-target areas. Drift severity is affected by numerous factors including droplet size, wind speed, application methods and settings (e.g. boom height), and the other ingredients in the formulation (Hock et al., 2016).
Drift retardants usually work by increasing average droplet size; however, this comes with tradeoffs. Larger droplets can reduce drift and enhance sticking, but they may reduce spread and contact area thereby lowering overall spray effectiveness. Still, the addition of drift control agents may be beneficial since drift also implies a loss as the spray doesn't do its intended purpose.
Thickeners increase the viscosity of the spray mix, which in effect causes larger droplet size. This can reduce drift by shortening the hang time of a droplet in the air. Thickeners (especially oils) also slow evaporation once a spray has been applied, which is important because many nutrient and pesticide solutions are only active when they are wet (Hock et al., 2016). Evaporation causes pesticides and nutrients to crystallize and become unavailable to plants. Thickeners may also alter the solubility of plant tissues aiding in the penetration of active ingredients, although the mechanisms are usually patented and proprietary information (Graber et al., 2015). Drift retardants and thickener products listed for organic production are usually oil- or lipid-based, and often derived from fish or plants.
OMRI/WSDA-listed examples: Drift Control and Thickeners
| Product | Manufacturer |
| Natur’l Oil | Stoller Enterprises |
| CNI Transcend | ChemNut, Inc. |
| Brandt A+ | Brandt |
| PHT Ad-Here SP Deposition Aid | JR Simplot |
Using an Adjuvant on Your Farm: Adjuvant Registration, Regulation, and Organic Compliance
The United States Environmental Protection Agency (EPA) is responsible for regulating and registering pesticides but regulates relatively few of the ingredients used as adjuvants, whether sold separately or in formulation. More recently, the NOP has taken over oversight of the EPA's List of Inerts of Minimum Concern. Some potential adjuvants may also appear on the FIFRA 25(b) List of Minimum Risk Pesticides that are also exempt from federal regulation.
Many adjuvants are considered inert ingredients, and therefore not required to be listed on labels by the federal government. Importantly, however, many adjuvants are biologically and chemically active compounds, and while some sources say they have "no pesticidal properties" (Hock et al., 2016), this is misleading if not inaccurate. In organic agriculture, many common adjuvants such as soaps and oils have well-demonstrated active pesticidal properties in research trials (Caldwell et al., 2013). In addition, there is growing concern about adjuvant toxicity and effects on non-target species like honeybees (for example, organosilicons have been shown to negatively affect honeybee learning) and fish (several types of nonionic surfactants and biosurfactants have been shown to cause reproductive mutations and embryonic toxicity in various species) (Mullin et al., 2016; Mesnage and Antoniou, 2018; Ciarlo et al., 2012)
While most adjuvants are exempt from federal regulation, 43 states do regulate materials on the FIFRA 25(b) list (Rodriguez et al., 2017). Additionally, nine states (AR, CA, ID, KY, MS, TN, UT, WA, and WY) currently require registration of adjuvants with their state departments of agriculture because adjuvants are included in their definitions of pesticide. In Washington State, for example, adjuvant registration requires a confidential declaration of all ingredients in the formulation, an adjuvant function statement that aligns with ASTM International (formerly American Society for Testing and Materials) standards, and efficacy data (G. Vetter, personal communication). California also requires efficacy data, but most other adjuvant-regulating states only require data on the potential impacts on groundwater quality (Rodriguez, 2017).
In addition, adjuvants must be approved for use on organic farms. Materials review organizations like OMRI (Organic Materials Review Institute) and WSDA (Washington State Department of Agriculture) go through the process of ensuring a product meets NOP standards. Inputs not listed by an input review organization can also be used on organic farms; to understand more about how to determine if an input can be used on organic farms, read the article Can I Use This Input on My Organic Farm? All products/materials used on an organic farm must be approved by the farm's certifier and written into the farm's Organic System Plan.
Choosing an Adjuvant
Admittedly, adjuvants are alluring for growers. Adjuvants offer an opportunity to save money, further reduce pests/pathogens, boost crop yields and quality, and mitigate environmental and human health effects of drift. Before setting out to include an adjuvant in a spray routine, a grower should know what they are trying to achieve with its addition. There are several considerations to think about before adding an adjuvant. Some of these are outlined in Table 2. There are many things to consider when choosing/seeking an adjuvant. These range from biological and environmental concerns to socio-political issues, and this list is not all-encompassing.
| Table 2: Some Factors to Consider when Choosing an Adjuvant |
| Adapted from Tu and Randall (2003) |
| Purpose |
| Prevention or control of specific pest |
| Environment |
| Site conditions (aquatic, sensitive area, neighboring farms) |
| Weather conditions (humidity, temperature, wind speeds) |
| Water chemistry (hard/soft, pH) |
| Target(s) and Host(s) |
| Species, type, biotype/cultivar (fungi, bacteria, weed, insect, etc.) |
| Phenological/Growth stage (disease cycle, newly sprouted) |
| Infestation severity (will it need high spray volume or high concentration?) |
| Barriers to spray (hairy/waxy leaves, canopy coverage) |
| Method of application (foliar spray, boom sprayer, soil drench, etc.) |
| (Economic) Values |
| Adjuvant efficacy |
| Adjuvant cost vs. benefits |
| Socio-political concerns |
| Logistics |
| Tank mixing order |
| Compatibility/interaction/phytotoxicity issues |
| Compliance |
| Compliance with organic regulations |
| Compliance with pesticide regulations |
Product labels should be the first stop for recommendations on appropriate adjuvants. Remember, many formulations come with an adjuvant(s) already added. The term emulsifiable can be a tipoff that a surfactant/adjuvant is already present. Pesticide labels may suggest which type of adjuvant to add (i.e., nonionic surfactant or spreader), or they might just generally suggest adding one. In some cases, a product may recommend a specific brand/product to add, although it is not clear if this is due to demonstrated synergy in controlled studies or because of market factors (e.g. the same company makes both the pesticide and the adjuvant and suggests buying their brand). It is also possible a product may specify to not add any adjuvant products. Pesticides and adjuvants must be used in accordance with all their label guidelines; not following the label or any non-labeled use of a pesticide/adjuvant is illegal. Luckily, most pesticides come with extensive label information including known phytotoxicities, known compatible/incompatible products, and recommended mixing orders. Labels may be lengthy and have small print (larger versions available online), and much of the information is extremely useful.
It would be nice if there was an easy and definitive way to determine which adjuvant is best for each grower/farm scenario, but as of yet, no such tool exists. Many references encourage growers to contact their local Extension office or weed specialists for recommendations. However, there are unfortunately few Extension agents with expertise in organic adjuvants. Notably, many organic-approved adjuvants are also used in conventional farming, although alongside synthetic pesticides.
Additionally, many organic adjuvant products are made from natural materials (e.g., fish or plant extracts) which makes them chemically diverse. In other words, they contain a mix of fatty acids and oils/lipids as opposed to a careful composition of pure ingredients in a more formulated product. This might be a good thing (because there is multi-functionality), but it makes prediction and specificity of an adjuvant's function more difficult.
A Realistic (but Hypothetical) Example of Using this Guide
Fred Waterford and his partner own and operate Sunny Harvest Farm in Newland, Kentucky, USA. In 2010, they began selling vegetables at their local farmers market. The following year, Sunny Harvest became USDA certified organic (the first in their part of Kentucky) and started a CSA. They began pasture-raising beef and pork to diversify their offerings in 2013. At Sunny Harvest, they pride themselves on producing a diversity of high-quality food with a business model that supports their community, their employees, and the environment.
To help cut down on food waste in their community, Sunny Harvest changed their CSA model in 2018 making it customizable for local buyers, who otherwise discarded the things they did not like. As such, heirloom tomatoes (already a high value crop for the farm) were one of the most in demand from local consumers. Fred is obviously interested in maximizing his tomato yields and quality, so he starts asking around for advice.
Fred likes to alternate spraying a preventative biologic (beneficial microbes that can compete with pathogenic ones) with a copper-based fungicide to deal with pesky early blight (fungus, Alternaria solani) in his fields. At the recommendation of his neighbor, a conventional farmer who also grows tomatoes, Fred began using a polymer-type adjuvant to help his sprays stick and remain active for longer periods of time. Unfortunately, he has noticed worsening tip burn since incorporating this new adjuvant. He is using the same rates and a similar fungicide (although organic approved) as his conventional neighbor who isn't seeing any leaf burn. Fred turns to this guide for help and comes up with the following:
- The neighbor recommending the adjuvant grows processing tomatoes, and Fred grows heirlooms—there can be varietal differences in the way plants respond to sprays and their ingredients (Czarnota and Thomas, 2013).
- The product Fred bought contains 70% proprietary polymer blend, 20% mineral oil, and 10% inert ingredients—oils (especially in hot climates like Kentucky) are known to cause phytotoxicity.
- Since adjuvants must be registered in Kentucky, the agriculture department can provide a shortlist of similar products registered for use in the state, so their ingredients can be compared.
- Fred finds a product with no added oils (just a polymer solution and water) and decides to try it.
- Fred works with his local Extension agent to create a plan for a paired comparison trial on his farm that will allow Fred to assess the results side by side: spray with no adjuvant vs. spray with polymer+oil adjuvant vs. spray with polymer-only adjuvant.
- Based on what Fred observes with the comparison, he can make a choice to switch (or drop) the adjuvant for his tomato sprays in the years to come.
The above example is based on a real farmer's predicament who reached out during the writing of this guide, although the names and details have been changed for privacy. For additional examples, see the University of Georgia Extension Bulletin #1319, which discusses a scenario where a grower chooses the wrong type of adjuvant and melts their greenhouse of pansy flowers. Admittedly, this guide has provided a lot of information on adjuvant function, sources, and chemistry, which can be overwhelming. Download the summary table at the end of this article, which summarizes this guide and provides an easy-to-use resource for growers interested in trying an adjuvant product. Due to the nature of adjuvant efficacy and all the factors that can affect a spray (environmental conditions, etc.), it is difficult if not impossible to give growers easy and direct recommendations/rules. So, while the guide can't give more specific recommendations, the information can be used to think more critically about what the best option(s) might be in different circumstances.
Download the adjuvant summary table here
References and Citations
- Arujo, J., J. Rocha, M. O. Filho, S. Matias, S. O. Junior, C. Padilha, and E. Santos. 2018. Rhamnolipids biosurfactants from Pseudomonas aeruginosa—A review. Biosciences Biotechnology Research Asia 15:4. (Available online at: http://dx.doi.org/10.13005/bbra/2685) (verified 19 Aug 2021).
- Berg, G., M. Koberl, D. Rybakova, H. Muller, R. Grosch, and K. Smalla. 2017. Plant microbial diversity is suggested as the key to future biocontrol and health trends. FEMS Microbial Ecology 93:5. (Available online at: https://doi.org/10.1093/femsec/fix050) (verified 19 Aug 2021).
- Borjesson, J. 2010. Enhancing deposition of agricultural sprays by the use of polymeric adjuvant MS Thesis. Chalmers University of Technology, Göteborg, Sweden. (Available online at: http://publications.lib.chalmers.se/records/fulltext/138788.pdf) (verified 19 Aug 2021).
- Brandt Consolidated. 2016. Adjuvant products guide. Brandt Consolidated, Inc., Springfield, IL. (Available online at: https://brandt.co/media/3682/brandt_adjuvants_guide.pdf) (verified 20 Aug 2021).
- Caldwell, B., E. Sideman, A. Seaman, A. Shelton, and C. Smart. 2013. Resource guide for organic insect and disease management. 2nd ed. Cornell University, Ithaca, NY. (Available online at: http://web.pppmb.cals.cornell.edu/resourceguide/) (verified 20 Aug 2021).
- Ciarlo, T. J., C. A. Mullin, J. L. Frazier, and D. R, Schmehl. 2012. Learning impairment in honey bees caused by agricultural spray adjuvants. PLOS One, 7(7), e40848. (Available online at: http://dx.doi.org/10.1371/journal/pone.0040848) (verified 20 Aug 2021).
- Czarnota, M., and P. A. Thomas. 2013. Using surfactants, wetting agents, and adjuvants in the greenhouse [Bulletin 1319]. University of Georgia Extension. (Available online at: https://extension.uga.edu/publications/detail.html?number=B1319&title=Using%20Surfactants,%20Wetting%20Agents,%20and%20Adjuvants%20in%20the%20Greenhouse) (verified 20 Aug 2021).
- Graber, E. R., Y. Elad, D. Rav David, and S. Segal. 2015. U.S. Patent No. 61753000. United States Patent and Trademark Office, Alexandria, VA.
- Hill, R. M. 1999. Siloxane surfactants. p. 1-48. In R. M. Hill (ed.), Silicone Surfactants. Marcel Dekker, New York, NY.
- Hock, W. K., K. H. Richards, S. I. Gripp, and B. Riden. 2016. Spray adjuvants. PennState Extension. (Available online at: https://extension.psu.edu/spray-adjuvants) (verified 20 Aug 2021).
- Hussain, M., B. Debnath, M. Qasim, B. S. Bamisile, W. Islam, M. S. Hameed, et al. 2019. Role of saponins in plant defense against specialist herbivores. Molecules 24:2067. (Available online at: http://dx.doi.org/10.3390/molecules24112067) (verified 20 Aug 2021).
- Jiang, X., H.C.B. Hansen, B. W. Strobel, and N. Cedergreen. 2018. What is the aquatic toxicity of saponin-rich plant extracts used as biopesticides? Environmental Pollution 236:416—424. (Available online at: http://dx.doi.org/10.1016/j.envpol.2018.01.058) (verified 20 Aug 2021).
- Lacaille-Dubois, M.-A., and H. Wagner. 2017. New perspectives for natural triterpene glycosides as potential adjuvants. Phytomedicine 37:49—57. (Available online at: http://dx.doi.org/10.1016/j.phymed.2017.10.019) (verified 20 Aug 2021).
- Liu, J.-F., S. M. Mbadinga, S.-Z Yang, J.-D Gu, and B.-Z Mu. 2015. Chemical structure, property and potential applications of biosurfactants produced by Bacillus subtilis in petroleum recovery and spill mitigation. International Journal of Molecular Sciences 16:4814—4837. (Available online at: http://dx.doi.org/10.3390/ijms16034814) (verified 20 Aug 2021).
- Madsen, M. D., E. G. Coronel, and B. G. Hopkins. 2013. Soil surfactant products for improving hydrologic function in post-fire water-repellant soil. Soil Science Society of America Journal 77:1825—1830. (Available online at: http://dx.doi.org/10.2136/sssaj2012.0305) (verified 20 Aug 2021).
- McClements, D. J. 2012. Nanoemulsions versus microemulsions: Terminology, differences, and similarities. Soft Matter 8:1719—1729. (Available online at: http://dx.doi.org/10.1039/C2SM06903B) (verified 20 Aug 2021).
- Mesnage, R., and M. N. Antoniou. 2018. Ignoring adjuvant toxicity falsifies the safety profile of commercial pesticides. Frontiers in Public Health 5:361. (Available online at: http://dx.doi.org/10.3389/fpubh.2017.00361) (verified 20 Aug 2021).
- Mullin, C. A., J. D. Fine, R. D. Reynolds, and M. T. Frazier. 2016. Toxicological risks of agrochemical spray adjuvants: Organosilicone surfactants may not be safe. Frontiers in Public Health 4:92. (Available online at: http://dx.doi.org/10.3389/fpubh.2016.00092) (verified 20 Aug 2021).
- Neri, D., E. M. Lodolini, M. Luciani, P. Sabbatini, and G. Savin. 2002. The persistence of humic acid droplets on leaf surface. Acta Horticulturae 594. (Available online at: http://dx.doi.org/10.17660/ActaHortic.2002.594.36) (verified 20 Aug 2021).
- Northover, J. and L. W. Timmer. 2002. p. 512-526. Control of plant diseases with petroleum and plant-derived oils. In Beattie, G. et al. (eds.) Spray Oils Beyond 2000. University of Western Sydney Press.
- Pacanoski, Z. 2015. Herbicides and adjuvants. p. 125—147. In L. Sarunaite, J. Kelton, and A. Price (eds.) Herbicides: Physiology of Action and Safety. InTech Open. (Available online at: http://dx.doi.org/10.5772/60842) (verified 20 Aug 2021).
- Rodriguez, A. C. 2017. Overview of the states pesticide registration process [Online slide presentation]. AAPCO Laboratory Committee Meeting. Available at: https://aapco.files.wordpress.com/2017/03/states-pesticide-registration-process-march-6-2017-aapco-meeting-acrodriguez-final-rev.pdf (verified 20 Aug 2021).
- Theodorakis, P. E., E. R. Smith, R. V. Craster, E. A. Muller, and O. K. Matar. 2019. Molecular dynamics simulation of the superspreading of surfactant-laden drops. A Review. Fluids 4:176. (Available online at: http://dx.doi.org/10.3390/fluids4040176) (verified 20 Aug 2021).
- Trece, Inc. 2012. CideTrakD Information Bulletin. Trece, Inc: Adair, OK. (Available online at: https://trece.com/wp-content/uploads/CIDETRAK-D-Information-Bulletin.pdf) (verified 20 Aug 2021).
- Tu, M., and J. M. Randall. 2003. Adjuvants. In Weed control methods handbook: Tools and techniques for use in naturalareas. The Nature Conservancy, Arlington, VA. (Available online at: https://www.invasive.org/gist/products/handbook/21.Adjuvants.pdf) (verified 20 Aug 2021).
- Zhu, Q. Q., C. Y. Shao, Z. Zhang, and X. H. Wen. 2010. Saponin biosurfactant-enhanced flushing for the removal of heavy metals from soils. Acta Scientiae Circumstantiae 30:2491—2498. (Available online at: https://www.researchgate.net/publication/288154871_Saponin_biosurfactant-enhanced_flushing_for_the_removal_of_heavy_metals_from_soils) (verified 23 Aug 2021).



