eOrganic authors:
Tariq Alam, Clemson University
Sachin Rustgi, Clemson University
Introduction
Bacterial wilt, also known as brown rot of potato, is caused by the group of soilborne bacteria in the Ralstonia solanacearum species complex. It is an economically significant disease of solanaceous vegetables, such as potato and tomato. The plant invasion of this soilborne pathogen is favored by high soil temperatures (above 85 ºF) and high soil moisture content. The bacterium enters the plant through natural openings, mechanical wounds, cracks, or root tips, then colonizes the cortex (sub-epidermal tissue) and infects the xylem vessels (conductive plant tissue) (Fig. 1). As the bacterium spreads in the xylem, it blocks the plant's water uptake, which leads to wilting and eventually death. After the plant dies, the pathogen remains in the plant debris and survives in the decaying plant matter. As the plant matter decays, the bacterium returns to the soil, where it can survive for several years in the absence of a host. This soil can serve as a primary source of new infections. If the soil is near or in a watercourse, it can also serve as a means of pathogen dissemination. The presence of root-knot nematodes in the field also exacerbates bacterial wilt infection, since they facilitate bacterial spread and root infiltration. Yields of tomato and potato plants that survive Ralstonia solanacearum infection drop significantly due to wilt.
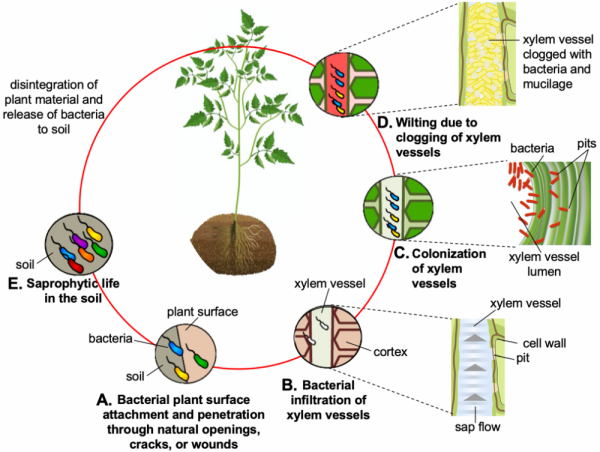
Figure 1. Different phases of the Ralstonia solanacearum life cycle, saprophytic (survives on soil organic matter in the absence of host) and pathogenic (feeds on the living host). A) Bacteria get attracted to the host root exudates, attach to the root surface, and penetrate roots via natural openings, cracks, and wounds. B) Bacteria make their way through cortex (the tissue beneath the skin) to the xylem vessels (the conductive tissue, through which the sap moves from ground to areal plant parts). C) Bacteria pass through the pits in the xylem vessel walls and colonize vessels. Inset shows the inside view of a xylem vessel with bacteria infiltrating through the pit to vessel lumen. D) Bacteria enter the vessels, populate them, and fill them with cells and mucilage (polysaccharides; see inset). This impairs the water flow leading to wilting. E) Due to excessive wilting, the host dies. With time the plant parts wither, and the bacteria release into the soil until a suitable host becomes available.
R. solanacearum is a genetically diverse pathogen with a vast host range (more than 400 host plant species), indicating many different genetic groups (strains and races). However, the disease severity conferred by each strain varies with the host. The length of time in which the bacteria can survive in the soil in the absence of a host is also variable and depends on several factors such as soil moisture, organic matter, and the availability of alternative hosts. Crop management solely via crop rotations is difficult, and an integrated disease management strategy is needed to control the bacterial wilt pathogen effectively.
Bacterial wilt is one of the most challenging diseases to manage in organic production. The following integrated disease management strategies should be adopted by organic farmers to effectively reduce the incidence of bacterial wilt in the host crops.
Disease Avoidance Strategies (Before Planting)
Scouting for disease symptoms. Accurate and early identification of the disease is vital for its management. Characteristic symptoms of R. solanacearum include wilting (i.e., drooping of plant parts due to insufficient water in plant body), foliar epinasty (i.e., downward curling of leaves due to the rapid cell growth on the upper side of the petiole), vascular discoloration of the stem to brown, and a light-brown vascular ring with ooze (in potato tubers) (see Fig. 2). For more information on R. solanacearum/bacterial wilt identification, field signs, and symptoms, consult this North Carolina State Extension publication (Meadows and Henson, 2017). It is important to recognize the difference between bacterial wilt, and fungal wilt caused by Verticillium and Fusarium species. The major differences in these wilts are: 1) The fungi proceed slowly in the host relative to bacteria and produce more uniform symptoms through the plant. 2) In bacterial wilt, symptoms appear from the top down, whereas in Fusarium and Verticillium wilt, symptoms begin at the bottom of the plant and progress upward. The biology of the causal organism and the host-pathogen interaction explains these visible differences. For example, in bacterial wilt, the impairment of water transport to areal plant parts due to clogging of the conductive plant tissue via rapid bacterial proliferation and mucilage production leads to wilting. In fungal wilt, production of toxins is the primary initial cause of wilting.
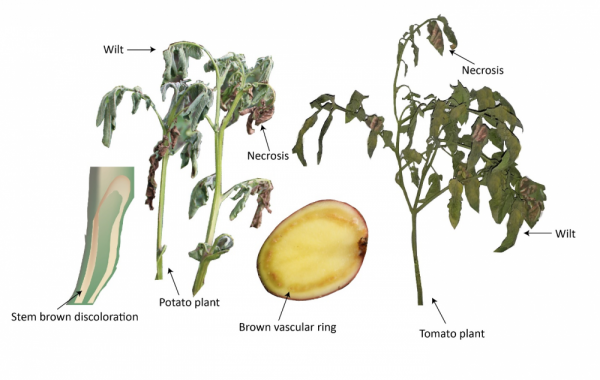
Figure 2. Characteristic symptoms of bacterial wilt of potato and tomato. Notice the browning of the conductive tissue in stem (closest to the "skin") and healthy pith (the inner tissue in the stem), when split lengthwise (extreme left). Potato plants showing the typical wilt symptoms with dead or necrotic leaves (left). Potato tuber cut open to show browning of the vascular tissue and masses of bacteria exuding from the cut ends of vascular strands. This browning is a diagnostic symptom of pathological wilting, which results from the oxidation of phenolic compounds produced during cell wall degradation (center). Tomato plant showing the typical wilt symptoms with dead leaves (right).
Site selection. Bacterial wilt is both a soilborne and a waterborne disease, meaning that the pathogen can survive in soil for up to two years after the crop harvest (Shamsuddin et al., 1978), and in water for up to four years (Alvarez et al., 2008; Hong et al., 2008) in the absence of a host. Therefore, if you have the option to select a site for tomato or potato cultivation: 1) Choose a field and neighboring area with no prior history of bacterial wilt disease. 2) Select land that is flat and well-drained. 3) Avoid areas that are free of the cross-flow of water from the other infected fields. 4) Avoid damp soils or areas with excessive moisture.
Elimination of the sources of infection. The pathogen may survive in vegetative propagules (potato tubers, tomato suckers), infected plant parts, alternate crop hosts (e.g. tobacco, eggplant, and pepper) and weed hosts (e.g. nightshade, stinging nettle), soil, irrigation water, contaminated farm tools, and equipment. Therefore, sanitizing harvesting tools and equipment before use is highly recommended. For good cleaning practices, see Cleaning and sanitizing tools, harvest containers, and surfaces.
Seed selection. Starting with commercially-available, clean, and certified organically-produced tomato/potato seed/tubers is an important strategy to manage bacterial wilt. The pathogen does not usually survive in dry tomato seeds; however, high seed moisture in potato tubers make survival and transmission possible. For more information on sourcing organic tomato seeds, see Sourcing Certified Organic Seed and the National Organic Program Regulations (Colley and Baker, 2015).
Seed treatment with hot air/water. Bacterial wilt can survive in potato seed tubers. Infected tubers should be disinfected by heat treatment. Bacterial wilt can be controlled by exposing the seed tubers to hot air (112 ºF) with 75% relative humidity for 30 min (Tsang et al., 1998). For information on hot water treatment of seed, see Keys to Disease Management in Organic Seed Crops and consult Johnson and Morton (2010). Producers can apply the heat treatment to the seeds in a seed preparation facility using a hot air blower and a humidifier or using hot water.
Resistant varieties. The most effective way to control bacterial wilt disease is to grow resistant varieties.
Tomato: There are some moderate and low-level bacterial-wilt-resistant commercial cultivars available to organic producers in the United States, which include the following:
| Cultivar | Resistance level | Reference |
| ‘Hawaii 7996’ | Moderate | Kwak et al. (2018) |
| ‘Venus’ | Moderate | Laterrot et al. (1978), Henderson and Jenkins (1972) |
| ‘Saturn’ | Moderate | Kaan et al. (1975), Henderson and Jenkins (1972) |
| ‘Caraibo’ | Moderate | Blancard (2012) |
| ‘King Kong’ | Moderate | Blancard (2012) |
| ‘BHN 466’ | Low | BHN Seeds, Bonita Spring, FL |
| ‘FL 7514’ | Low | Champoiseau and Momol (2009) |
| ‘Neptune’ | Low | Pradhanang et al. (2005) |
Potato: There is only one bacterial wilt resistant potato cultivar available to organic producers, namely Red Pearl (University of Wisconsin, Madison; Groza et al., 2004). The authors are unaware if non-treated seeds of Red Pearl are available for organic production. However, the variety was tested successfully under plasticulture in Pennsylvania (visit the PennState Extension website for details).
Grafting onto resistant rootstocks. Bacterial-wilt-resistant tomato rootstocks have been developed and could be used in fields infested by the wilt pathogen to reduce disease pressure. For more information on the acquisition of the tomato-wilt-resistant rootstock, see Vegetable Grafting.
| Rootstock cultivar | Resistance level | Developer |
| ‘RST-04-111-E’ | Near complete resistance | DP Seeds, Yuma, AZ |
| ‘RST-05-113-TE’ | Near complete resistance | DP Seeds, Yuma, AZ |
| ‘RST-04-105-T’ | Complete resistance | DP Seeds, Yuma, AZ |
| ‘RST-04-106-T’ | Complete resistance | DP Seeds, Yuma, AZ |
Plant spacing. Increasing row spacing can reduce wilt incidence in soil with low pathogen populations. The recommended plant spacing to reduce wilt incidence is 1.5 to 2.5 feet.
Soil and rhizome solarization. Solarization of the soil before planting with transparent plastic mulch for 60 days can reduce wilt incidence (Vinh et al., 2005). These mulches can be applied in the field by hand or mechanically. Soil solarization used in combination with biological control agents (BCAs) such as Pseudomonas spp., Bacillus spp., and Streptomyces spp. (growth-promoting bacteria) reduces tomato wilt incidence more efficiently (Anith et al., 2000; Kumar et al., 2001; Chen et al. 2013; Yuliar et al., 2015; Marian et al., 2019).
Strategies to Use During the Crop Growing Season
Clean irrigation water. The bacterial wilt pathogen is waterborne, enabling it to multiply and disseminate via irrigation water. Organic farmers should make sure that irrigation water is clean and free from infestations. For irrigation water treatment, readers are encouraged to visit these publications to learn how to reduce disease pressure by treating drip irrigation systems with chlorine and testing water purity (Water Research Center webpage).
Soil amendments and fertilization. Amending soil with stable bleaching powder and lime has been shown to effectively control or slow down bacterial wilt (Dhital et al., 1997; Kishore et al., 1996). There are many synthetic and non-synthetic substances used as bleach that are allowed in organic production systems with some restrictions (visit the OMRI webpage for further details). Mature compost mixed with amino acid fertilizer (organic fertilizer) has also been shown to reduce bacterial wilt incidence (Ding et al., 2013); however, not all composts are disease suppressive.
IMPORTANT: Before using any disease control product in your organic farming system:
- Read the label to be sure that the product is labeled for the crop and disease you intend to control, and make sure it is legal to use in the state, county, or other location where it will be applied.
- Read and understand the safety precautions and application restrictions.
- Make sure that the brand name product is listed in your Organic System Plan and approved by your USDA-approved certifier. If you are trying to deal with an unanticipated problem, get approval from your certifier before using a product that is not listed in your plan—doing otherwise may put your certification at risk.
Note that, although OMRI and WSDA lists are good places to identify potentially useful products, all products that you use must be approved by your certifier. For more information on how to determine whether a disease control product can be used on your farm, see the eOrganic article, Can I Use This Input On My Organic Farm?
Weed management. Weeds (nightshade, stinging nettle), and volunteer tomatoes and potatoes act as hosts to bacterial wilt. Therefore, managing weeds and destroying volunteer plants before transplanting can help reduce disease pressure.
Crop rotation. Rotation with non-host crops is considered one of the most important disease management strategies in organic agriculture. The incidence of bacterial wilt disease can be reduced by regular rotations with non-host crops such as corn, sorghum, wheat, carrots, cowpea, and soybean. Crop rotation also offers several other advantages, such as the maintenance of soil structure and organic matter. A two- to five-year rotation or fallowing for about a year with frequent disking during the dry season is generally recommended to eradicate the wilt bacterium (Shamsuddin et al., 1978).
Harvest. Potato: Avoid wet harvest conditions, since moist conditions can exacerbate potato tuber damage and the spread of disease. The roots and stems of infected crops should be destroyed after harvest to help prevent further contamination of the soil.
Harvest tool and equipment sanitization. Bacterial wilt may spread through contaminated farming tools and equipment. Therefore, sanitize harvesting tools and equipment before use. For more information on cleaning harvesting equipment, see the video Cleaning Harvest Equipment prior to sanitizing.
Storage. Bacterial wilt symptoms can appear in potatoes during storage. To prevent this, store healthy potatoes at low temperatures (less than 50 ºF) and scout them for the appearance of wilt symptoms. Collect and eliminate infected potato tubers.
Acknowledgements
This work was supported by the NIFA Hatch/Multi-state grant (S009).
Bibliografia
- Álvarez, B., M. M. López, and E. G. Biosca. 2008. Survival strategies and pathogenicity of Ralstonia solanacearum phylotype II subjected to prolonged starvation in environmental water microcosms. Microbiology 154:3590—3598. (Available online at: https://doi.org/10.1099/mic.0.2008/019448-0) (verified 17 Mar 2023).
- Anith, K. N., T. P. Manomohandas, M. Jayarajan, K. Vasanthakumar, and K. C. Aipe. 2000. Integration of soil solarization and biological control with a fluorescent Pseudomonas sp. for controlling bacterial wilt Ralstonia solanacearum (EF Smith) Yabuuchi et al. of ginger. Journal of Biological Control 14:25—29. (Available online at: http://www.informaticsjournals.com/index.php/jbc/article/view/4020 (verified 17 Mar 2023).
- Blancard, D. 2012. Tomato diseases: Identification, biology and control. 2nd ed. Page 552. Manson Publishing, London, England.
- Champoiseau, P., Timur M. Momol. 2009. Bacterial wilt of tomato [Online]. Available at https://plantpath.ifas.ufl.edu/rsol/Trainingmodules/BWTomato_Module.html (Verified 17 Mar 2023).
- Chen, Y., F. Yan, Y. Chai, H. Liu, R. Kolter, R. Losick, and J.-H. Guo. 2013. Biocontrol of tomato wilt disease by Bacillus subtilis isolates from natural environments depends on conserved genes mediating biofilm formation. Environmental Microbiology 15:848—864. (Available online at: https://doi.org/10.1111/j.1462-2920.2012.02860.x (verified 17 Mar 2023).
- Colley, M., B. Baker. 2015. Sourcing certified organic seed and the national organic program regulations [Online]. eOrganic article. Available at: https://eorganic.org/node/2304 (Verified 17 Mar 2023).
- Dhital, S. P., N. Thaveechai, W. Kositratana, K. Piluek, and S. K. Shrestha. 1997. Effect of chemical and soil amendment for the control of bacterial wilt of potato in Nepal caused by Ralstonia solanacearum. Kasetsart Journal, Natural Sciences 31:497-509.
- Ding, C., Q. Shen, R. Zhang, and W. Chen. 2013. Evaluation of rhizosphere bacteria and derived bio-organic fertilizers as potential biocontrol agents against bacterial wilt (Ralstonia solanacearum) of potato. Plant and Soil 366:453—466. (Available at: https://doi.org/10.1007/s11104-012-1425-y) (verified 17 Mar 2023).
- Groza, H. I., B. D. Bowen, D. Kichefski, S. J. Peloquin, and J. Jiang. 2004. Red pearl: A new gourmet red potato variety. American Journal of Potato Research 81:209—213. (Available online at: https://link.springer.com/article/10.1007%2FBF02871751 (verified 17 Mar 2023).
- Hong, J. C., M. T. Momol, J. B. Jones, P. Ji, S. M. Olson, C. Allen, A. Perez, P. Pradhanang, and K. Guven. 2008. Detection of Ralstonia solanacearum in irrigation ponds and aquatic weeds associated with the ponds in North Florida. Plant Disease 92:1674—1682. (Available online at: https://doi.org/10.1094/PDIS-92-12-1674) (verified 17 Mar 2023).
- Johnson. K., F. Morton. 2010. Keys to disease management in organic seeds crops [Online]. eOrganic Article. Available at: https://eorganic.org/node/2777) (Verified 17 Mar 2023).
- Kaan F., H. Laterrot, G. Anaïs. 1975. Etude de 100 variétés de tomate en fonction de l‟adaptation climatique et de la résistance à sept maladies sévissant aux Antilles. ouvelles Agronomiques desAntilles et de la Guyane, 1 (2), 123—138. (Available online at: https://hal.inrae.fr/hal-02731297/document) (verified 17 Mar 2023).
- Kishore, V., G. S. Shekhawat, and V. Sunaina. 1996. Cultural practices to reduce Pseudomonas solanacearum in the infested soil. Journal of the Indian Potato Association 23:130—133.
- Kumar, P., and A. K. Sood. 2001. Integration of antagonistic rhizobacteria and soil solarization for the management of bacterial wilt of tomato caused by Ralstonia solanacearum. Indian Phytopathology 54:12—15. (Available onlinel at: http://epubs.icar.org.in/ejournal/index.php/IPPJ/article/view/18831) (verified 17 Mar 2023).
- Kwak, M. J., H. G. Kong, K. Choi, S. K. Kwon, J. Y. Song, J. Lee, E. J. Jung, H. Park. N. Roy, H. Kim, M. M. Lee, E. M. Rubin, A. W. Lee, and J. K. Kim. 2018. Rhizosphere microbiome structure alters to enable wilt resistance in tomato. Nature Biotechnology 36:1100—1109. https://doi.org/10.1038/nbt.4232) (verified 17 Mar 2023).
- Laterrot, H., J. F. Kaan. 1978. Resistance to Corynebacterium michiganense of lines bred for resistance to Pseudomonas solanacearum. Report of the Tomato Genetics Cooperative 28:7—8. University of California, Davis. (Available online at: https://tgc.ifas.ufl.edu/onlinevo.htm) (verified 17 Mar 2023).
- Marian, M., A. Morita, H. Koyama, H. Suga, and M. Shimizu. 2019. Enhanced biocontrol of tomato bacterial wilt using the combined application of Mitsuaria sp. TWR114 and nonpathogenic Ralstonia sp. TCR112. Journal of General Plant Pathology 85:142–154. (Available online at: https://doi.org/10.1007/s10327-018-00834-6) (verified 17 Mar 2023).
- Meadows. I., M. Henson. 2017. Southern bacterial wilt of tomato [Online]. North Carolina State Extension. Available at: https://content.ces.ncsu.edu/bacterial-wilt-of-tomatoes (Verified 17 Mar 2023).
- Nolte, K. D. 2014. Cleaning harvest equipment prior to sanitizing [Online]. University of Arizona. Available at: https://www.youtube.com/watch?v=YdzPzz6E60k (Verified 17 Mar 2023).
- Oram, B. 2014. C-T contact time and inactivation calculations for chlorine disinfection [Online]. Water Research Center. Available at: https://water-research.net/index.php/water-treatment/water-disinfection/chlorine-disinfection (Verified 17 Mar 2023).
- Schonbeck, M. 2019. Twelve steps toward ecological weed management in organic vegetables [Online]. eOrganic Article. Available at: https://eorganic.org/node/2320 (Verified 17 Mar 2023).
- Schermann, M., A. Hultberg. 2018. Cleaning and sanitizing tools, harvest containers and surfaces [Online]. University of Minnesota. Available at: https://extension.umn.edu/growing-safe-food/cleaning-and-sanitizing-tools-harvest-containers-and-surfaces (Verified 17 Mar 2023).
- Shamsuddin, N., A. B. Lloyd, and J. Graham. 1978. Survival of the potato strain of Pseudomonas solanacearum in soil bacterial wilt; New South Wales. Journal of the Australian Institute of Agricultural Science.
- Storlie C. 1997. Treating drip irrigation systems with chlorine [Online]. New Jersey Agricultural Experiment Station, Rutgers. Available at: https://njaes.rutgers.edu/FS795/ (Verified 17 Mar 2023).
- Tsang, M. M. C., and M. Shintaku. 1998. Hot air treatment for control of bacterial wilt in ginger root. Applied Engineering in Agriculture 14:159—163. (Available at: https://doi.org/10.13031/2013.19365) (verified 17 Mar 2023).
- USDA Specialty Crops Research Initiative, Vegetable Grafting Research Based Information Portal. 2020. Commercial solanaceous rootstocks. [Online]. Available at: http://www.vegetablegrafting.org/resources/rootstock-tables/solanaceous-rootstocks/ (Verified 17 Mar 2023).
- Vinh, M. T., T. T. Tung, and H. X. Quang. 2005. Primary bacterial wilt study on tomato in vegetable areas of Ho Chi Minh City, Vietnam. p. 177—184. In C. Allen, P. Prior, and A. C. Hayward (eds.). Bacterial Wilt Disease and the Ralstonia solanacearum species complex. American Phytopathological Society Press, St. Paul, MN.
- Wei, Z., J. F. Huang, J. Hu, Y. A. Gu, C. L. Yang, X. L. Mei, Q. Shen, Y. Xu, and V. P. Friman. 2015. Altering transplantation time to avoid periods of high temperature can efficiently reduce bacterial wilt disease incidence with tomato. Plos One 10(10):e0139313. (Available online at: https://doi.org/10.1371/journal.pone.0139313) (verified 17 Mar 2023).
- Wisconsin Alumni Research Foundation. Potato line W84-75R ("Red Pearl") [Online]. University of Wisconsin—Madison. Available at: https://plantbreeding.wisc.edu/about/germplasm-developed/potato/ (Verified 17 Mar 2023).
- Yuliar, Y. A. Nion, and K. Toyota. 2015. Recent trends in control methods for bacterial wilt diseases caused by Ralstonia solanacearum. Microbes and Environments 30:1—11. (Available online at: https://www.jstage.jst.go.jp/article/jsme2/30/1/30_ME14144/_article) (verified 17 Mar 2023).
Additional Resources
- Champoiseau. P. G., J. B. Jones, T. M. Momol, P. Ji, C. Allen, D. J. Norman, C. Harmon, S. A. Miller, T. Schubert, D. Bell. D., J. P. Floyd, D. Kaplan, R. Bulluck, K. Smith, and K. Cardwell. 2010. Ralstonia solanacearum race 3 biovar 2 causing brown rot of potato, bacterial wilt of tomato and southern wilt of geranium [Online]. Available at: https://www.ars.usda.gov/ARSUserFiles/00000000/opmp/RalstoniaR3b2May2010.pdf (Verified 17 Mar 2023).



