eOrganic authors:
Leigh Archer, University of Florida Department of Horticultural Sciences
Dean Watson, University of California Department of Plant Pathology
Louise Ferguson, University of California Department of Plant Sciences
Amanda Hodson, University of California Department of Entomology and Nematology
Contact: larcher1@ufl.edu; (401)256-4156; 2685 SR 29 N Immokalee, FL 34142
Introduction
California olive oil production has been expanding rapidly over the past two decades, due in part to the selection of varieties that respond well to mechanical pruning and over-the-row harvesting, such as the cultivar 'Arbequina'. Marketing efforts have also resulted in a strong expansion in the domestic demand for olive oil. While many regions of California have excellent potential for olive oil production, the presence of the soilborne pathogen causing Verticillium wilt is a limiting factor in the expansion of orchards into some areas. Olives are particularly susceptible to this disease. Although some organic pre-plant management options are available, such as solarization, these are not commonly employed and only occasionally provide effective disease suppression. Additionally, there are no highly effective post-plant management strategies for either conventional or organic growers.
Understanding Verticillium dahliae
Verticillium wilt (VW) is a vascular disease caused primarily by the soil fungal pathogen Verticillium dahliae. Verticillium wilt can affect a wide range of plants. At least 400 plant species are hosts and the fungus forms survival structures, microsclerotia, which can remain viable in the soil for over ten years (Baroudy et al., 2018). There are few viable options for control, especially for organic growers. Pre-plant solarization may be able to reduce disease pressure initially, but this is strategy rarely provides long-term efficacy for perennial crops.
The disease survives in the soil until the microsclerotia germinate due to nearby root activity. After the microsclerotia germinate, they transition to a parasitic phase. Fungal hyphae grow through the roots into the xylem, and the fungus is carried through the plant with the xylem fluid. The fungal growth reduces water transport within the plant, causing wilt, defoliation, and other visible symptoms of decreased water availability (Keykhasaber et al., 2017; Santos-Rufo, 2017).
Verticillium Wilt in Olives
Verticillium wilt has emerged as one of the most serious problems facing olive production worldwide. In the United States, the disease was first detected in California olive trees in the 1950s and quickly spread throughout the state (Wilhelm et al., 1962). Verticillium wilt often occurs in orchards that are started in fields previously planted in susceptible crops (Keykhasaber et al., 2017), such as cotton or tomato. These crops are common in the areas of California where olive oil producers are looking to expand their acreage. The recent trend of planting olives at high or super-high densities can also increase the chances of infection because more trees produce more roots within the same soil volume, increasing the likelihood that the fungus will be activated (Rodriguez et al., 2008).
Genetic resistance to the VW fungus has been tested across a variety of olive cultivars and genotypes with little success (Lopez-Escudero et al., 2004). The majority of the more commonly planted cultivars are highly susceptible, including the widely-planted oil cultivar in California 'Arbequina', and no cultivar has been found that is entirely resistant (Hegazi et al., 2012; Leyva-Perez et al., 2017). The most common recommendation for VW in olives is to avoid planting in areas where the pathogen has been detected, as pre-plant strategies rarely eliminate the microsclerotia population and there are no effective recommendations for post-plant management.
Current Verticillium Wilt Management Options
Previously reported successful conventional VW control combined soil fumigation with other pre-plant measures to reduce soil inoculum levels. However, these relied heavily on chemicals, and few have been successful in perennial systems (Gomez-Galvez and Rodriguez-Jurado, 2018).
A variety of organic soil amendments, such as composts or mulches, have been suggested as possible options for combatting VW-infested soil (Termorshuizen et al., 2007). These amendments may provide some level of biological control by reducing the microsclerotia concentration in the soil (Termorshuizen et al., 2007). The severity of VW is closely linked to the fungal population density in the soil, so reducing the soil microsclerotia levels could decrease the disease pressure, particularly for perennial species.
The fungus is commonly spread through irrigation water. Over the life span of an orchard there are constantly new opportunities for infestation, which increases the risk of infestation compared to annual crop systems. This continual pressure means orchard growers cannot rely exclusively on pre-plant measures. It is critical to create a soil environment inhospitable to the fungus by applying and incorporating amendments, which is difficult when trying to avoid disrupting the root system of perennial plants. Resources with additional information on management options are included in the References section of this document.
Olive Mill By-Products
Modern mills rely on machinery to complete the oil extraction process. Olives are mechanically cleaned and crushed, and the resulting paste is mixed before the oil is separated from the pomace by centrifugation (Camposeo and Vivaldi, 2013). Technological advances have increased efficiency and oil mill volume, increasing oil mill pomace to environmentally critical levels (Camposeo and Vivaldi, 2013). Pomace is the sludgy organic material consisting of ground olive fruits and pits that remain after the oil extraction. This product is phytotoxic, and although the toxicity is caused by naturally occurring organic compounds, proper management is critical to avoid pollution of the surrounding ecosystems (Yakhlef et al., 2018). Many mills dispose of their pomace, sell it as animal feed, or compost the raw product to remove the phytotoxic compounds (Ceglie et al., 2011).
Experimental trials have also indicated that pomace may be useful in an integrated soil management plan (Aviles and Borrero, 2017). Incorporating raw olive pomace into the soil may provide growers with a natural, organically-approved weed control option for inhibiting seed germination of the field's weed seed bank, and reducing growth of the pathogen causing VW. These benefits underwent preliminary testing in the laboratory using in vitro analysis of fungal suppression and weed seed germination, as well as in the greenhouse using field soil that had been inoculated with the fungus causing VW.
IMPORTANT: Before using any pest or disease control product in your organic farming system:
- Read the label to be sure that the product is labeled for the crop and pest you intend to control, and make sure it is legal to use in the state, county, or other location where it will be applied.
- Read and understand the safety precautions and application restrictions.
- Make sure that the brand name product is listed in your Organic System Plan and approved by your USDA-approved certifier. If you are trying to deal with an unanticipated pest problem, get approval from your certifier before using a product that is not listed in your plan—doing otherwise may put your certification at risk.
Note that, although OMRI and WSDA lists are good places to identify potentially useful products, all products that you use must be approved by your certifier. For more information on how to determine whether a pest control product can be used on your farm, see the article, Can I Use This Input On My Organic Farm?
Verticillium Wilt Management with Olive Pomace: Results from In Vitro Analysis
The naturally-occurring toxic elements in olive pomace may provide organic growers an option for VW management, though it is unclear whether the phenolic compounds, tannins, long-chain fatty acids, lignins, or other compounds contribute most strongly toward VW suppression. Both raw and composted pomace significantly reduced the growth of the fungus causing Verticillium wilt. These results were visible in cultures inoculated with the VW fungus and treated with different concentrations of raw pomace and composted pomace extract (Figs. 1 & 2).
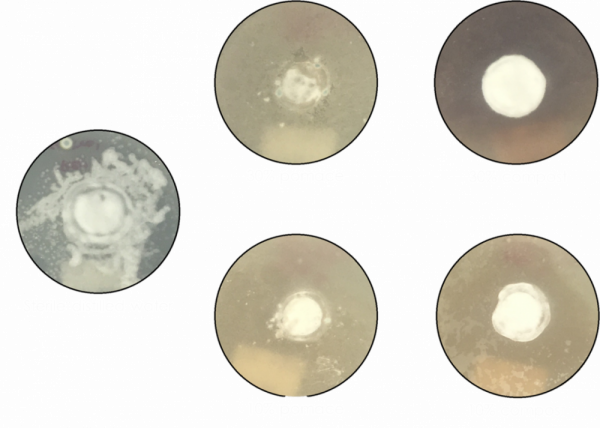
Figure 1. Images showing the growth of white V. dahliae hyphae in culture plates. Untreated fungi (left) compared to pomace extract treated fungi (left two columns) compared to composted pomace extract treated fungi (right). Photo credit: Leigh Archer, University of Florida.
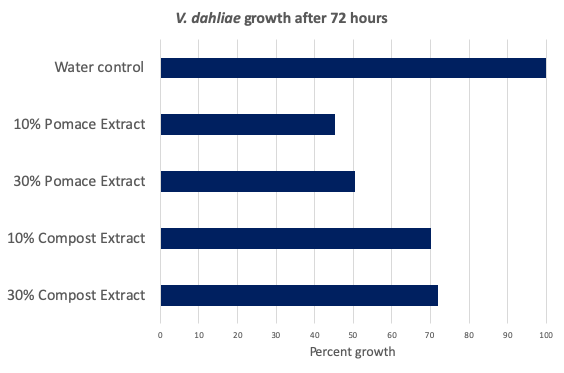
Figure 2. Fungal growth as a percent of untreated control after 72 hours when treated with 10% or 30% dilution of raw pomace or composted pomace extracts. Extracts were a filtered 1:1 (w:w) dilution of sterile distilled water and material. Raw pomace extract reduced fungal growth by 50%, significantly more than the reduction from the composted product. From six replicates, both compost and pomace extracts were significantly lower than the control (p<.001). Both pomace concentrations were also significantly lower than both compost concentrations (p<.05). Data were analyzed using Microsoft Excel and R Software. Statistical tests performed included analyses of variance and, when treatment differences were significant, a pairwise comparison of estimated marginal means using the Tukey method.
In addition to lab experiments, a greenhouse experiment using field soil taken from an organic olive orchard was artificially inoculated with the VW fungus and then treated with raw olive pomace. Six replicate soil samples were mixed with a cornmeal-sand inoculum at a low (5% w/w) or high (20% w/w) rate before being thoroughly mixed with raw olive pomace. Six weeks after inoculation, soil samples diluted in water were plated on a selective medium to estimate the level of fungal infestation with and without pomace treatment based on a visual ranking of percent coverage of each plate. The amendments did not completely eradicate the fungus, however there was a significant decrease in infestation with the addition of the raw pomace (Fig. 3). Given the suppressive nature of the raw pomace, incorporation of the material into the soil following milling each year may be useful as a preventative strategy by maintaining the soil in a condition less hospitable to fungal growth.
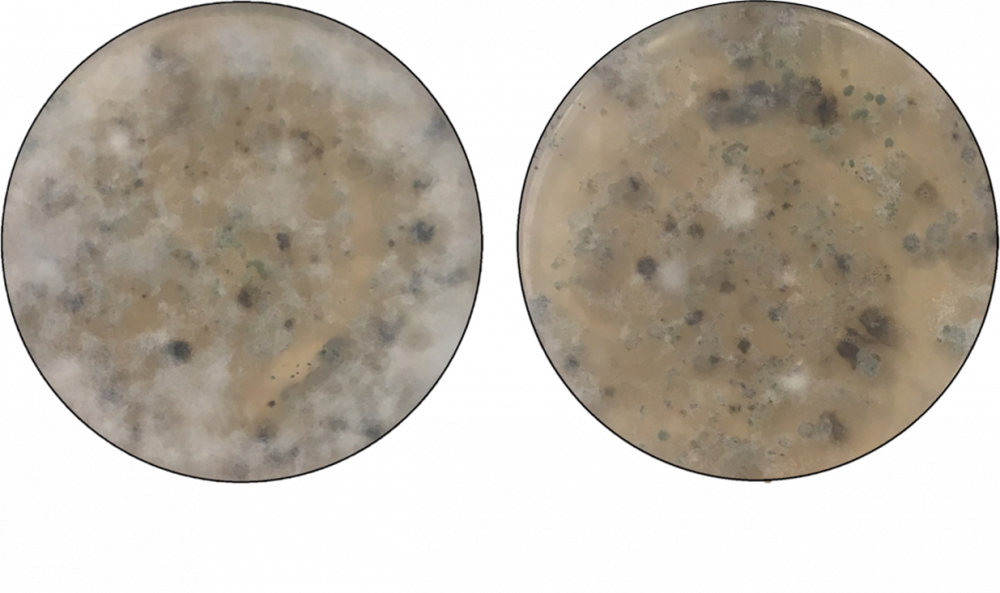
Figure 3. Image of the reduced infestation and vigor of the fungus causing Verticillium wilt in untreated soils (left) compared to pomace treated soils (right). White fluffy hyphal masses are the VW hyphae. Photo credit: Leigh Archer, University of Florida.
Based on these results, amending soil with raw olive pomace for VW suppression before planting an orchard may provide some level of disease suppression. Pre-plant incorporation could reduce the population of the fungus in the soil, but will likely not completely eliminate it. Using olive pomace as one part of an integrated management strategy will likely lead to the best results. As these are only preliminary laboratory results, these potential benefits will need to be confirmed with larger-scale field testing using different application rates and timings. Additional pre-plant management tactics can be found in this newsletter. (Note: this newsletter was not reviewed by eOrganic for National Organic Program certification compliance).
Weed Management with Olive Pomace
A common metric for determining the phytotoxicity of a soil amendment or compound is the germination index. (Zucconi et al., 1981). The germination percentage and root elongation of seeds treated with an amendment is compared to untreated seeds. Raw olive pomace significantly decreased the germination index at 3 different concentrations (Figs. 4 & 5), and the germination and elongation of pomace-treated cress seeds was consistently less than 3% of the untreated seeds.
Composted olive pomace products were also tested for phytotoxicity by determining the germination index. The two olive pomace composts did not significantly decrease germination and root growth of seeds treated compared to the untreated seeds, and in some cases the composts actually enhanced growth. This suggests the composted product is not phytotoxic (Ceglie et al., 2011), even at concentrations of 50%, which would be well above a grower standard application rate for compost.
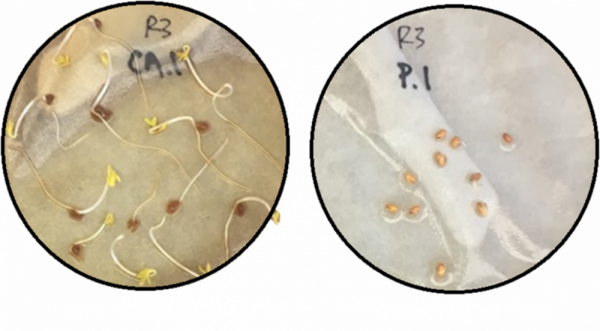
Figure 4. Image of phytotoxicity testing after 72 hours. Seeds treated with composted pomace (left) compared to seeds treated with raw pomace (right). Photo credit: Leigh Archer, University of Florida.
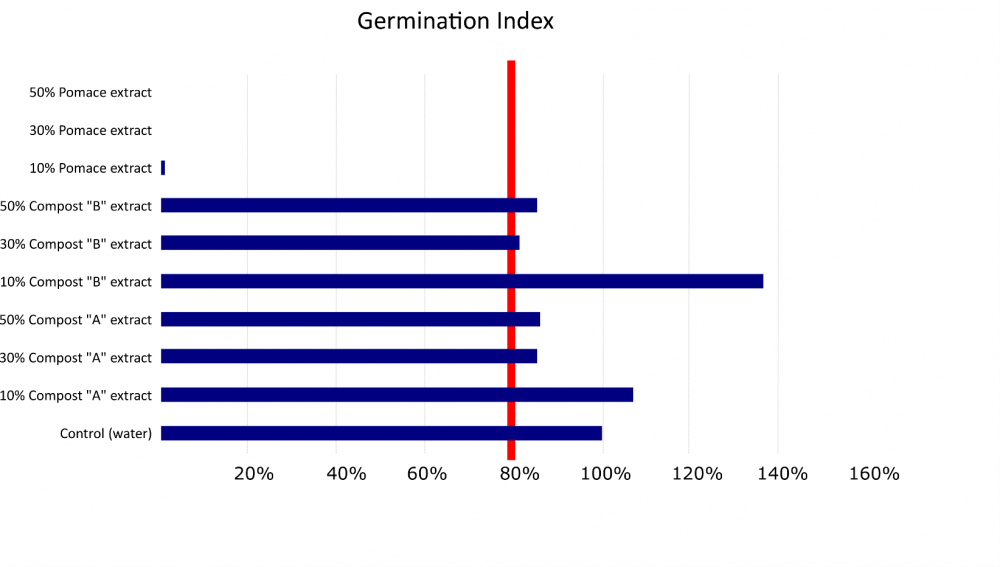
Figure 5. Phytotoxicity testing results based on the germination index (relative root growth x relative seed germination). Three concentrations of raw olive pomace treated seeds compared to 6 olive pomace compost treated seeds and untreated control seeds. The two compost mixes tested varied in the proportion of different drying agents added to the raw pomace; these included almond hulls, green waste, and orchard prunings. Products are considered phytotoxicity-free when the germination index exceeds 80% (red line).
A complementary greenhouse trial looked at the impact of young olives grown in pomace-treated soil by measuring tree height, trunk diameter, leaf relative water content, photosynthesis, and total leaf area. There was no difference in any of these parameters for the pomace-treated trees compared to control trees after 18 weeks (unpublished data), results supported by previous studies (Nasini et al., 2013; Camposeo and Vivaldi, 2011). Olives could be naturally adapted to grow in soil environments rich in the compounds found in pomace, which could explain why the phytotoxic compounds in pomace did not significantly impact tree growth. As suggested by the results of the phytotoxicity testing, raw pomace has potential for organic weed control by inhibiting germination. Some California organic olive growers have anecdotally expressed success with pomace for weed control, however, the proper rates of application should be determined on an individual basis depending on the tree age, soil characteristics, and other management practices of the orchard.
Given initial results from a greenhouse trial that suggest olives are unharmed by the compounds in the pomace, it is possible that other tree crop or annual crop species may exhibit a similar resistance. If applied as a pre-plant amendment in orchards or annual crops with enough time to decompose in order to minimize phytotoxic compounds, olive pomace could be a weed suppressant in a variety of other organic production systems. Application of the pomace may be challenging on a large scale because of its consistency and weight. Additional technologies and methods would need to be adopted in order to evenly apply the product if weed management proves effective in the field.
Potential for Using Olive Pomace
The use of raw olive pomace is a high priority for the olive mills. To obtain the potential benefits of olive pomace for weed suppression or fungal management, it would be of greatest value if the product is applied in its raw form. Currently, most mills need to dispose of pomace in a manner that minimizes potential environmental damage from the natural toxicity of the product. Pomace can be composted to remove phytotoxic elements, however this significantly increases costs and requires storage space. Pomace is a fairly wet product, so composting requires the addition of drying materials such as green waste or almond hulls. Application of raw pomace directly after milling eliminates the need for space and the processing time required to create compost, and eliminates the need for proper environmentally-sound pomace disposal.
In addition to reducing waste, raw pomace may be useful for organic growers as weed or VW control. Raw pomace and its extracts were extremely effective at reducing seed germination and reducing the spread and vigor of Verticillium dahliae in laboratory and greenhouse analyses. Field studies to determine the appropriate rates, timing, and application methods may provide additional insights and recommendations for growers interested in utilizing the by-product of the oil milling process.
References and Citations
- Aviles, M., and C .Borrero. 2017. Identifying characteristics of Verticillium wilt suppressiveness in olive mill composts. Plant Disease 101:1568—1577. (Available online at: https://doi.org/10.1094/PDIS-08-16-1172-RE) (verified 27 Aug 2020).
- Baroudy, F., W. Habib, G. Tanos, E. Gerges, C. Saab, E. Choueiri, and F. Nigro. 2018. Long-distance spread of Verticillium dahliae through rivers and irrigation systems. Plant Disease 102:1559—1565. (Available online at: https://doi.org/10.1094/PDIS-08-17-1189-RE) (verified 27 Aug 2020).
- Camposeo, S., and G. A. Vivaldi. 2013. Short-term effects of de-oiled olive pomace mulching application on a young super high-density olive orchard. Scientia Horticulturae 129:613—621. (Available online at: https://doi.org/10.1016/j.scienta.2011.04.034) (verified 27 Aug 2020).
- Ceglie, F. G., H. Elshafie, V. Verrastro, and F. Tittarelli. 2011. Evaluation of olive pomace and green waste composts as peat substitutes for organic tomato seedling production. Compost Science & Utilization 19:293—300. (Available online at: https://doi.org/10.1080/1065657X.2011.10737011) (verified 27 Aug 2020).
- Gómez-Gálvez, F. J., and D. Rodríguez-Jurado. 2018. Potential efficacy of soil-applied disinfectant treatments against Verticillium wilt of olive. Crop Protection 106:190—200. (Available online at: https://doi.org/10.1016/J.CROPRO.2018.01.002) (verified 27 Aug 2020).
- Hegazi, H. A. 2012. Performance of 12 introduced olive cultivars under Egyptian conditions. Journal of Agriculture and Biological Sciences 8:98—107. (Available online at: http://www.aensiweb.net/AENSIWEB/rjabs/rjabs/2012/98-107.pdf) (verified 27 Aug 2020).
- Keykhasaber, M., K.T.K. Pham, B.P.H.J. Thomma, and J. A. Hiemstra. 2017. Reliable detection of unevenly distributed Verticillium dahliae in diseased olive trees. Plant Pathology 66:641—650. (Available online at: https://doi.org/10.1111/ppa.12647) (verified 27 Aug 2020).
- Leyva-Pérez, M. O., J. Jiménez-Ruiz, C. Gómez-Lama Cabanás, A. Valverde-Corredor, J. B. Barroso, F. Luque, and J. Mercado-Blanco. 2018. Tolerance of olive (Olea europaea) cv Frantoio to Verticillium dahliae relies on both basal and pathogen-induced differential transcriptomic responses. New Phytologist 217:671—686. (Available online at: https://doi.org/10.1111/nph.14833) (verified 27 Aug 2020).
- López-Escudero, F. J., C. del Río, J. M. Caballero, and M. A. Blanco-Lopez. 2004. Evaluation of olive cultivars for resistance to Verticillium dahliae. European Journal of Plant Pathology 110:79—85. (Available online at: https://doi.org/10.1023/B:EJPP.0000010150.08098.2d) (verified 27 Aug 2020)
- Nasini, L., G. Gigliotti, M. A. Balducini, E. Federici, G. Cenci, and P. Proietti. 2013. Effect of solid olive-mill waste amendment on soil fertility and olive (Olea europaea L.) tree activity. Agriculture, Ecosystems & Environment 164:292—297. (Available online at: https://doi.org/10.1016/j.agee.2012.10.006 (verified 27 Aug 2020).
- Rodríguez, E., J. M. García-garrido, P. A. García, and M. Campos. 2008. Agricultural factors affecting Verticillium wilt in olive orchards in Spain. European Journal of Plant Pathology 122:287—295. (Available at: https://doi.org/10.1007/s10658-008-9287-0) (verified 27 Aug 2020).
- Santos-Rufo, A., V. Vega, J. J. Hidalgo, and D. Rodriguez-Jurado. 2017. Assessment of the effect of surface drip irrigation on Verticillium dahliae propagules differing in persistence in soil and on Verticillium wilt of olive. Plant Pathology 66:1117—1127. (Available online at: https://doi.org/10.1111/ppa.12652) (verified 27 Aug 2020).
- Termorshuizen, A. J., E. van Rijn, D. J. van der Gaag, C. Alabouvette, Y. Chen, J. Lagerlof, A. A. Malandrakis, E. J. Paplomatas, B. Ramert, J. Ryckeboer, C. Steinberg, and C. Zmora-Nahum. 2006. Suppressiveness of 18 composts against 7 pathosystems: Variability in pathogen response. Soil Biology and Biochemistry 38:2461—2477. (Available online at: https://doi.org/10.1016/J.SOILBIO.2006.03.002) (verified 27 Aug 2020).
- Wilhelm, S., W. J. Kaiser, S. G. Georgopoulus, and K. W. Opitz. 1962. Verticillium wilt of olives in California. Phytopathology 52:32 (abstract).
- Yakhlef, W., R. Arhab, C. Romero, M. Brenes, A. de Castro, and E. Medina. 2018. Phenolic composition and antimicrobial activity of Algerian olive products and by-products. LWT-Food Science and Technology 93:323—328. (Available online at: https://doi.org/10.1016/J.LWT.2018.03.044 (verified 27 Aug 2020).
- Zucconi, F., M. Forte, M. Monaco, M. De Bertoldi, A. P. Monaco, and M. Bertoli. 1981. Biological evaluation of compost maturity. Biocycle 22:27—29.
Additional Resources
- Dung, J.K.S. 2020. Verticillium wilt in the Pacific Northwest. 2020 Pacific Northwest Plant Disease Management Handbook. Oregon State University. (Available online at: https://pnwhandbooks.org/plantdisease/pathogen-articles/common/fungi/verticillium-wilt-pacific-northwest (verified 27 Aug 2020).
- Vossen, P., D. Gubler, and M. A. Blanco. 2008. Verticillium wilt of olive. University of California Cooperative Extension. (Available online at: http://cesonoma.ucanr.edu/files/27504.pdf) (verified 27 Aug 2020).



