eOrganic author:
David D. Douds Jr., USDA-ARS Eastern Regional Research Center
Introduction
Arbuscular mycorrhizal (AM) fungi are naturally occurring soil fungi that colonize the roots of most crop plants and form a symbiosis. There are benefits to the plant and fungus, as well as to the soil in general, that result from the symbiosis. Improved uptake of immobile soil mineral nutrients, water relations, and disease resistance are among the benefits to plants credited to the AM symbiosis. The fungi benefit through the capture of sugars within the root. Ecosystem benefits include increased stability of soil aggregates. Given these benefits, utilization of the AM symbiosis should be an important tool in sustainable agricultural systems—especially organic systems, which prohibit the use of synthetic fertilizers and pest control.
There are two opportunities for the utilization of AM fungi. The first is to manage effectively the indigenous population already present on the farm. This will be the topic of another article. The second is to inoculate your plants with effective isolates of AM fungi. This article will discuss the on-farm production and utilization of inoculum of AM fungi.
A hard-to-understand, and even harder to override, characteristic of AM fungi limits the ways in which inoculum may be produced. Arbuscular mycorrhizal fungi are believed to be obligate symbionts, that is they must colonize plant roots to grow and reproduce. Only the phase of the fungus inside the root (“intraradical hyphae”) can absorb sugar and express certain metabolic pathways necessary for growth, such as the synthesis of fats. Therefore, the fungus has a very limited ability to grow asymbiotically, i.e. without living in symbiosis. Failure by researchers to overcome these limitations has prohibited the growth of these organisms in pure culture on Petri dishes or in fermenters for inoculum production.
Inocula of AM fungi currently are produced in a variety of ways utilizing laboratory, greenhouse, or field-based methods. The most effective laboratory method is in Petri dishes with carrot root organ cultures. Greenhouse methods include classical pot culture in which AM fungus spores are inoculated into greenhouse pots in which host plants are grown. Inocula produced by these methods are commercially available. The costs of these methods—for example, greenhouse or lab space/time/labor, isolation of the AM fungi from the original media and/or mixing with a carrier substrate—as well as shipping and handling costs, must be borne by the farmer. On-farm production of inocula avoids many of these costs and could make this symbiosis, and the associated economic and environmental benefits, available to more farmers. In addition, these methods can produce inoculum containing the indigenous AM fungi already adapted to one’s farm.
On-farm Production of AM Fungus Inoculum
General considerations
An important consideration in AM fungus production is the level of available P in the media in which the plant hosts are grown. Plants growing in high P situations limit colonization of their roots by AM fungi. In effect, they are deciding to limit the “cost” (in terms of sugar) of the symbiosis in the absence of benefit (in this case, improved uptake of phosphorus) since the roots can function well enough on their own in the high nutrient situation. The reduction of colonization then limits the fungus’ ability to acquire sugars for growth and reproduction. This phenomenon is also important in the greenhouse production phase for the growth of inoculated vegetable seedlings, as we will see later.
There are several factors to consider when selecting a host plant for the AM fungi. Obviously, one must select a plant that supports colonization by AM fungi. Though most dicotyledonous plants become colonized by AM fungi, certain families such as the mustards and other crops such as spinach and sugar beet do not become colonized by AM fungi and should not be used as host plants. Good hosts are typically members of the grass family, for example corn, sorghum-sudangrass, and others. Secondly, one must consider the eventual crop plant that will be inoculated, and choose a host plant for inoculum production from a different family. Plants of the same family are more likely to share susceptibility to pests or pathogens and one does not want to unintentionally inoculate the crop with a pest. Lastly, the ideal host plant also will not become a weed pest later on. One should choose a host that is not hardy in your area or be sure to remove fruiting structures before they mature.
A final consideration is to guard against the possibility of propagating harmful pathogens within your inoculum production system. Use clean seed of the selected host plant and a propagation medium you trust.
We will now discuss on-farm production of AM fungus inoculum. After a brief summary of methods used by researchers in tropical areas, we will outline the method developed for temperate climates and how the above considerations were addressed.
On-farm production of inoculum in tropical areas
On-farm production of AM fungus inoculum in Columbia and India has been described previously. Sieverding (1991) developed a procedure in Colombia whereby a starter inoculum of an AM fungus was introduced into raised beds of fumigated soil. After four months of growth of host plants, 42 to 96 spores cm-3 were produced in the soil. Only 9 spores cm-3 were produced without fumigation.
Another method was developed at the Tata Energy Research Institute in India (Gaur, 1997). In this method, starter inoculum of AM fungi was mixed into furrows in raised beds of fumigated soil. Three crops, e.g. sorghum, corn, and carrot, were grown successively in the bed, each for three months. The soil and roots were chopped and remixed prior to planting the next nurse host. This method produced an average of 22.7 infectious propagules cm-3 after three one-year cycles. A modification of this method using one of three vegetable crops as a host plant produced approximately one propagule cm-3 in soil amended 1:1 or 1:2 (v/v) with compost (Gaur et al., 2000).
On-farm Inoculum Production in Temperate Climates
General procedure
We have developed a method for the on-farm production of AM fungus inoculum in temperate climates that requires no input of synthetic chemicals or fumigation (Douds et al., 2005, 2006). Bahiagrass (Paspalum notatum Flugge) seedlings, colonized by AM fungi, are transplanted into black plastic bags containing a mixture of compost and vermiculite. The plants are tended over the growing season (weeded and watered as needed) and the AM fungi proliferate as roots spread throughout the compost and vermiculite mixture. Frost then kills the bahiagrass. The AM fungi naturally overwinter in the media and the inocula are ready for use the following spring. This system has successfully propagated all AM fungi tested, and produced hundreds of propagules cm-3 in a 1:4 [v/v] mixture of yard clippings compost and vermiculite (Douds et al., 2005).
Materials
- weed barrier cloth (e.g. American Agrifabrics, Alpharetta, GA 33003)
- seven gallon black plastic “grow bags” (Worm’s Way, Bloomington, IN 47404)
- vermiculite
- compost
- conical plastic pots, 66 cm3 (RLC-4 Pine Cell, Stuewe and Sons, Inc., Corvallis, OR 97333)
Host plant
We have chosen bahiagrass as the host plant for AM fungus inoculum production for a number of reasons. First, it has been shown in greenhouse experiments to be a reliable host for the majority of AM fungi tested. Secondly, since the inoculum production system targets primarily vegetable producers, the use of a graminaceous host plant is warranted because of the lack of crop plants from this family among common vegetables. Lastly, being a tropical C4 grass, it will be frost killed so as to not become a weed pest. Our experience with nearly 8 years of work with the on-farm system in southeastern PA has shown only occasional survival of bahiagrass usually from underneath the bags of inoculum.
Production of colonized seedlings
When we developed this procedure, we envisioned that bahiagrass seedlings colonized by specific species of AM fungi would become available commercially. Though this may eventually happen, a grower interested in producing inoculum can instead seek to propagate the AM fungi indigenous to his/her farm. This can be done by collecting soil from a field not recently used to grow the eventual crops to be inoculated (see the general considerations, above) or from a natural area on the farm such as a wood lot or fence row. The use of soil from a native, undisturbed plant community on your farm is especially attractive since it may contain the diverse, healthy soil biological community present before the impacts of often decades of agricultural management. Since the majority of AM fungus propagules in the field are in the top 10 cm of soil, just use that layer. Sieve out stones and roots, and mix this soil 1:1 or 1:2 with sand (volume basis) and place the mixture in the conical plastic pots. Transplant bahiagrass seedlings into the pots and grow for 3 months or so. Keep the pots weeded to avoid introducing weeds to the eventual inoculum. The roots of bahiagrass will become well colonized during this time and plants then will be ready for transplant into the inoculum production system. These plants will be the “nurse host” plants for the propagation of the AM fungi, native to your farm, that were present in the soil used.
The inoculum production system
Prepare a small plot in an area of the farm that receives full sun and that is conveniently near a water source. Cover the ground with the weed barrier fabric. The black plastic bags containing the compost and vermiculite mixture will be placed on this surface.
| Compost type | |||
|---|---|---|---|
| Characteristic | Yard Clippings | Dairy Manure + Leaf | Controlled Microbial |
| pH | 7.7 | 8.0 | 8.1 |
| Total N | 2.0% | 2.1% | 1.3% |
| Phosphorus | 0.27% | 0.47% | 1.04% |
| Potassium | 1.00% | 1.11% | 1.70% |
| C:N | 13.1:1 | 15.9:1 | 9.3:1 |
| N:P | 7.4:1 | 4.5:1 | 1.25:1 |
1 Analyses were conducted by the Penn State Agricultural Analytical Services Laboratory, University Park, PA 16802. Dry weight basis, elemental N, P, and K.
Maximal production of inoculum in this system requires the proper dilution of the nutrient rich compost with a nutrient poor substrate such as vermiculite. This is because colonization of roots by AM fungi, and hence growth of the fungus, is inhibited by high nutrient levels, notably of available P (see the general considerations, above). We did try growing the bahiagrass plants in pure compost one year and saw no spread of colonization within the root system or spore production by AM fungi. Further, the extent of the dilution will vary for composts with different nutrient levels. Table 1 shows important characteristics of three composts studied in the development of this technology. We conducted a complete factorial experiment (all possible combinations were examined) with three AM fungi, three composts, and five dilution ratios. Inoculum production was quantified in terms of spore production by the fungi (Fig. 1). Notice how spore production in the composts with the lower P levels and high N:P ratios (yard clippings compost and dairy manure plus leaf compost) was better at lower dilutions while that in the compost with high P and low N:P ratio (controlled microbial compost) was better when the compost was diluted more. We have developed predictive equations (Douds et al., 2008) in which compost nutrient levels are used to estimate compost and vermiculite dilutions for the growth of three AM fungi. In general, however, we have had success routinely with dilutions ratios of 1:3 to 1:9 (1:4 typically) compost:vermiculite, volume basis.
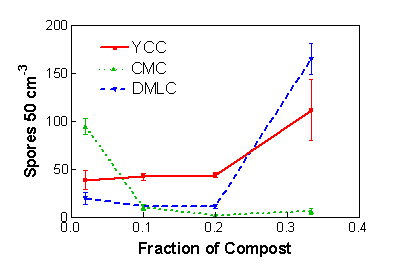
Figure 1. Spore production by the AM fungus Gigaspora rosea in mixtures of compost and vermiculite. YCC= yard clippings compost, DMLC= dairy manure + leaf compost, CMC= controlled microbial compost. Means of three observations ± SEM. Figure credit: David D. Douds Jr., USDA-ARS Eastern Regional Research Center.
In addition to providing the nutrients needed for the plants during the entire inoculum production period, compost provides another important benefit. The possibility that we may do something to introduce pathogens into the rhizosphere of seedlings in the greenhouse is a cause for lost sleep. We have yet to see this happen, due in part to the pathogen suppressive qualities of compost and our choice of host plant.

Figure 2. On-farm inoculum production bags showing recently transplanted bahiagrass seedlings. Figure credit: David D. Douds Jr., USDA-ARS Eastern Regional Research Center.
When the day comes to set up the system—that is, after the threat of frost has passed and your inoculated seedlings have been growing for a few months—fill the black plastic bags with your compost and vermiculite mixture and transplant the colonized bahiagrass seedlings into the bags (4–5 seedlings per bag) (Fig. 2). Water thoroughly. Throughout the growing season, monitor the bags weekly, and water and weed as necessary. This may take 5–10 minutes per week. No supplemental nutrient addition has been necessary. You should notice a lush growth of foliage later in the season (Fig. 3).

Figure 3. On-farm inoculum production bags showing late summer abundant growth of bahiagrass. Figure credit: David D. Douds Jr., USDA-ARS Eastern Regional Research Center.
As winter approaches, frost will kill the bahiagrass. There is no need to do anything further. Spore production by the AM fungi peaks at about this time as their hosts die. The bags are left outdoors, and the AM fungi overwinter in place. The inoculum is harvested for use the following spring.
A step-by-step summary of the procedure, to keep handy as you work through this, is given in Appendix I.
Utilization of the Inoculum
In general, crop plants may be inoculated with AM fungi one of two ways. Plants may be inoculated in the field at planting, or seedlings produced in the greenhouse for later outplanting may be grown in inoculated potting media. We have targeted vegetable growers who produce their own seedlings for outplanting because we feel the inoculum can be easily and economically mixed into potting media. Potential benefits to crop growth arise from the advantage the plant receives from having a pre-established symbiosis upon entering the field rather than experiencing the lag time before being colonized by the indigenous AM fungi.
Utilizing the inoculum now requires three steps and a little decision making: harvest of the inoculum, mixing into the potting media, and finally, potentially modifying one’s greenhouse nutrient regime.
Harvest of the inoculum
There are three types of infectious propagules of AM fungi: spores, pieces of colonized roots, and viable hyphae. All three are produced in the on-farm system.
Spore production will vary depending upon species in your indigenous AM fungus community and how close you came to an optimal compost and vermiculite mixture. The routine 1:4 ratio, yard clippings compost:vermiculite, produced an average of 30 spores cm-3 of mixture (Douds et al., 2006). The easiest way to harvest the spore plus viable hyphae fraction of the inoculum is simply to remove the root ball from the bags and shake the media off of the roots into a large bin. You may want to remove the dead leaves first to avoid them getting into the inoculum.
The bahiagrass roots are another source of inoculum. Most AM fungi produce structures called “vesicles” within the roots. These are spore like, globular organs that are reservoirs of fats (energy storage). The fungus is capable of regrowth in the spring out from dead root pieces that contain vesicles. Therefore, the root pieces may be chopped up fresh from the bag into small pieces and mixed with the inoculum. Bahiagrass roots are routinely 70 to 80% root length colonized by AM fungi in this system (Douds et al., 2006), so one may be confident that small pieces will contain AM fungi.
Mixing inoculum into the potting media
The next real decision-making step occurs when it is time to mix the inoculum into the potting media. There are two things to consider here: the potency of your inoculum, and the rooting volume of the cells in which the plants will be grown. Three years of on-farm production of AM fungi at 7 cooperating farms as part of a SARE grant yielded an average of 82 ± 20 propagules of AM fungi cm-3 (SARE grant LNE 03-179 final report). Propagule numbers averaged 503, 240, and 42 propagules cm-3 for the 1:4, 1:9, and 1:99 mixtures of yard clippings compost and vermiculite (Douds et al., 2006). Though this indicates only several cm3 of inoculum would be needed per planting cell to reach a target of 100-200 propagules of AM fungi per plant, it is very difficult to uniformly mix the inoculum into the potting media at a rate of only 1-2% by volume.
We recommend utilizing the inoculum at a dilution of 1:9 or 1:19 (inoculum:potting media, volume basis). This comes as a result of an experiment conducted with 8 cultivars each of tomato and pepper. Plants were grown in a greenhouse for 4 weeks in 50-cell flats (70 cm3 per cell). Two sets of plants were grown in horticultural potting media: one amended with inoculum at a 1:9 and the other at a 1:19 (inoculum:potting media, volume basis) dilution ratio. The initial, pure inoculum had a propagule density of 120 propagules cm-3. At the end of the experiment, tomato plants averaged 30.5% and 12.9% root length colonized by AM fungi for the 1:9 and 1:19 dilutions, respectively. Pepper averaged 14.8% and 8.0% root length colonized. All of these colonization intensities are sufficient to potentially produce a growth response. Choice between the 1:9 or 1:19 amendment would depend upon individual flat cell size. Cells that are 50 cm3 or smaller should be filled with media amended 1:9 with inoculum to ensure proper mixing and sufficient number of propagules.
After the seedlings have been transplanted into the inoculated potting media, the next decision is proper choice of fertilization regime, especially for conventional growers. With too much phosphorus, plants will not become colonized and the inoculum—or the money used to purchase the inocula—will have been wasted. To aid the conventional grower in this decision, we conducted an experiment to describe the response of AM fungus colonization of tomato and pepper roots to added P. Plants received, three times per week, 10 mL of a balanced, complete nutrient solution (Hoagland and Arnon, 1938) adjusted to supply P levels ranging from 0.31 to 62 ppm P as KH2PO4. Nitrogen was supplied as KNO3 and Ca(NO3)2 and was 210 ppm for all treatments. Results underscored the need to control P applications and showed that colonization decreased with increasing P level to effectively zero at 32 ppm P (Fig. 4).
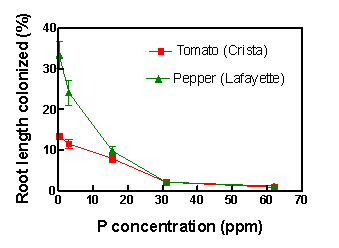
Figure 4. Percentage root length colonized by AM fungi of tomato cv. Crista and pepper cv. Lafayette. Plants were fertilized three times per week with a nutrient solution containing varying levels of P for six weeks. Means of seven observations ± SEM. Figure credit: David D. Douds Jr., USDA-ARS Eastern Regional Research Center.
Organic growers must find a different strategy to adjust P availability in their potting media. We have conducted experiments on this topic, but as yet only preliminary information is available (Table 2). We utilized an organically approved potting mix (NP mix from Living Acres, New Sharon, ME) with an NPK analysis of 0.4–0.5–0.3. The manufacturer recommends no nutrient addition to young plants grown in this media in the greenhouse. However, since P appeared to be high relative to N, we added two further treatments to the experiment. In addition to growing plants in media amended only with the inoculum, we grew plants in the media mixed 1:1 with vermiculite with and without a supplemental low P fertilizer (“Biogrow,” 1.8–0.1–6.6 from Biobizz, Groningen, The Netherlands). Ten mL of a stock solution containing 11.67 mL of the concentrated Biogrow per L was applied three times each week for 5 weeks. This supplied the same amount of supplemental N as was given in the inorganic treatments in the previous work (Figure 4). The goal of this work is to develop a regime in which plants are sufficiently colonized by AM fungi and of a size that is competitive with plants grown in high P. The results were mixed, and appeared to vary by cultivar (Table 2). Diluting the potting medial with vermiculite tended to decrease shoot growth, and this was alleviated by the extra nutrient addition. The effect upon colonization by AM fungi was inconsistent, but told us we are headed in the right direction. However, colonization of plants grown in the unamended media was still better than we have found in conventionally managed greenhouses, so we recommend this to growers as a place to start.
| Crop/cultivar | Treatment | Height (cm) | Shoot Wt. (g) | Colonization (% root length) |
|---|---|---|---|---|
| Tomato cv. Brandywine | ||||
| NP mix | 30.3 a | 0.876 a | 3.1 a | |
| NP:Verm 1:1 | 23.2 b | 0.701 a | 6.4 a | |
| NP:Verm+ Biogrow | 28.5 a | 0.809 a | 4.1 a | |
| Tomato cv. Paragon | ||||
| NP mix | 20.9 a | 1.041 a | 0.9 b | |
| NP:Verm 1:1 | 19.9 a | 0.746 b | 4.6 a | |
| NP:Verm+ Biogrow | 23.4a | 0.882 ab | 5.6 a | |
| Pepper cv. Colossal | ||||
| NP mix | 20.8 a | 0.666 a | 5.8 a | |
| NP:Verm 1:1 | 17.8 b | 0.565 a | 5.9 a | |
| NP:Verm+ Biogrow | 17.5 b | 0.613 a | 13.9 a | |
| Pepper cv. Lafayette | ||||
| NP mix | 23.7 a | 0.901 a | 2.9 a | |
| NP:Verm 1:1 | 18.8 b | 0.706 ab | 2.1 a | |
| NP:Verm+ Biogrow | 18.2 b | 0.572 b | 2.1 a | |
1 NP mix (organic potting media, 0.4–0.5–0.3 [N:P2O5:K2O] from Living Acres, New Sharon, ME 04955), Verm (vermiculite), Biogrow (1.8–0.1–6.6 from Biobizz, Groningen, The Netherlands). Means of six observations, numbers in the same column, within a cultivar, followed by the same letter are not significantly different (Tukey’s Method of Multiple Comparisons a = 0.05).
Concluding Remarks
In developing a system for the on-farm production of AM fungus inoculum, the goal of our research was to make a potent, effective, species diverse inoculum that was also very inexpensive. Our research examining the yield response of crops inoculated with AM fungi, in high P soils under both conventional and organic management, has shown highly significant increases some years, and little or no response in other years (Douds and Reider, 2003; Douds et al., 2007). However, routine use of this inoculum should not be an economic burden during years of optimal conditions in which inoculation gives no response yet positions one to take advantage of the potential for a significant yield response when the symbiosis alleviates conditions that depress yield.
Acknowledgements
This work was supported in part by a grant from the USDA-ARS CSREES Sustainable Agriculture Research and Education Program (LNE 03-179).
Disclaimer: Mention of a brand or firm name does not constitute an endorsement by the US Department of Agriculture over others not mentioned.
References and Citations
- Douds, D. D., and C. Reider. 2003. Inoculation with mycorrhizal fungi increases the yield of green peppers in a high P soil. Biological Agriculture and Horticulture 21: 91–102.
- Douds, Jr., D. D., G. Nagahashi, P. E. Pfeffer, W. M. Kayser, and C. Reider. 2005. On-farm production and utilization of mycorrhizal fungus inoculum. Canadian Journal of Plant Science 85: 15–21. (Available online at: http://pubs.aic.ca/doi/abs/10.4141/P03-168) (verified 21 June 2015).
- Douds, Jr., D. D., G. Nagahashi, P. E. Pfeffer, C. Reider, and W. M. Kayser. 2006. On-farm production of AM fungus inoculum in mixtures of compost and vermiculite. Bioresource Technology 97: 809–818. (Available online at: http://dx.doi.org/10.1016/j.biortech.2005.04.015) (verified 10 March 2010).
- Douds, Jr., D. D., G. Nagahashi, C. Reider, and P. R. Hepperly. 2007. Inoculation with arbuscular mycorrhizal fungi increases the yield of potatoes in a high P soil. Biological Agriculture and Horticulture 25: 67–78. Available online at: https://pubag.nal.usda.gov/catalog/21020 (verified 10 Oct 2019).
- Douds, D. D., G. Nagahashi, C. Reider, and P. Hepperly. 2008. Choosing a mixture ratio for the on-farm production of AM fungus inoculum in mixtures of compost and vermiculite. Compost Science and Utilization 16: 52–60.
- Gaur, A. 1997. Inoculum production technology development of vesicular-arbuscular mycorrhizae. Ph. D. thesis. Univ. of Delhi, Delhi, India.
- Gaur, A., A. Adholeya, and K. G. Mukerji. 2000. On-farm production of VAM inoculum and vegetable crops in marginal soil amended with organic matter. Tropical Agriculture 77: 21–26.
- Hoagland, D. R., and D. I. Arnon. 1938. The water-culture method for growing plants without soil. University of California College of Agriculture, Agriculture Experiment Station Circular 347. Berkeley, CA.
- Sieverding, E. 1991. Vesicular-arbuscular mycorrhiza management in tropical agrosystems. Deutsche Gesellschaft für Technische Zusammansabeit (GT2) GmbH. Eschbon, Germany.
Additional Resources
- Smith, S. E., and D. J. Read. 2008. Mycorrhizal symbiosis, 3rd Ed. Academic Press, San Diego, CA.
- International culture collection of (vesicular) arbuscular mycorrhizal fungi [Online]. College of Agriculture, Forestry and Consumer Sciences, West Virginia University. Available at: https://invam.wvu.edu/ (verified 10 Oct 2019).
Appendix I. Checklist of steps for the on-farm production of arbuscular mycorrhizal [AM] fungus inoculum.
The target date for setting up the system is as early as possible after the last frost. Some work is necessary before this date, and the following list is designed to help you get things started so everything is ready on time.
4 months before the predicted date of the last frost
- Germinate bahiagrass seeds in a pot of vermiculite or seed starter. Sources of seed can be found on the internet, for example: http://agriseek.com/market/p/Pensacola-Bahiagrass-Seed.htm
- Purchase materials
- Conical plastic pots (we use RLC-4 Pine Cells from Stuewe and Sons, Corvallis, OR 97333)
- 7 gallon “Grow Bags” (one source is Worm’s Way, Bloomington, IN 47404)
- Ground cover fabric
3 months before the predicted last frost
- Collect soil from 0 to 10 cm (4 in) depth from a natural area on the farm or a field that has not been used within the past 2 years to grow the crops to be inoculated. This is to avoid introducing pathogens.
- Mix this soil with sand (1:3 soil:sand, volume basis) and use the mixture to fill the conical plastic pots. We use a coarse sand, such as swimming pool filter sand.
- Transplant bahiagrass seedlings into the pots, fertilize occasionally with a low-P or P-free fertilizer if necessary. The plants will become colonized by the AM fungi native to your soil.
As soon as possible after the last frost
- Set up the inoculum production area:
- Cover an area with the ground cover fabric (16 bags fit nicely on a plot 1.2 m x 3.6 m (4 ft x 12 ft)
- Mix compost with vermiculite. A good general recommendation is 1:4 compost:vermiculite (volume basis) using yard clippings compost produced by municipal composting facilities. Fill the 7 gallon bags approximately ¾ full with the mixture. Roll the lip of the bags down to just above the level of the mixture. Sixteen bags require approx. four 4 ft3 bags of vermiculite and 4 ft3 of compost.
- Transplant 5 bahiagrass plants into each bag.
For the remainder of the growing season
- Weed and water the bags as needed. The AM fungi will proliferate as the plants grow.
Frost will kill the bahiagrass. The mycorrhizal fungi will overwinter naturally outdoors in the bags. The following spring when you are producing your seedlings in the greenhouse:
- Harvest the inoculum. Shake the compost and vermiculite mixture free from the roots into a collection bin. If you remove most of the dead leaves prior to this step, the resulting inoculum will be cleaner. Mix the inoculum from several bags together. This mix will contain spores produced by the fungi and living hyphae. You may also chop the roots into short segments (less than 1 cm [1/2 inch]) and mix these in as well.
- Mix the inoculum into your potting media. For flats with cells of 50 cm3 or less, use a 1:9 (inoculum: media, volume basis) mixture. For larger cells, a 1:19 mixture should be sufficient.
- Greenhouse fertilization regime. This aspect of the process is very important. Add too much phosphorus and the plants will resist colonization by the fungi, and all of your work to this point will have been just practice.
- Conventional farms. Remember the discussion earlier in this article: try to achieve a P addition of 15 ppm or less for no more than three fertilizer applications per week. Apply P-free solutions at other times if necessary.
- Organic farms. If your potting media requires additional fertilization, use a low P source. If your potting media contains all the nutrients needed during the greenhouse culture phase, no modifications are recommended at this time.



